July 7th, 2012
New Algorithm for Patients with LBBB and Suspected MI
John W McEvoy, MB BCh BAO, Ian Neeland, MD, James De Lemos, MD and John Ryan, MD
CardioExchange editor John Ryan interviews John W. McEvoy, cardiology fellow at Johns Hopkins, about an algorithm — newly proposed by Ian Neeland and colleagues in JACC — for selecting patients with left bundle-branch block and suspected MI for primary PCI. Neeland and coauthor James de Lemos respond to each of McEvoy’s comments.
The Algorithm
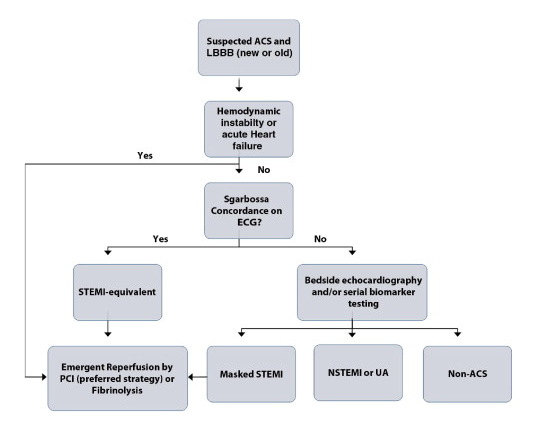
In their JACC Viewpoint article, Neeland and his colleagues highlight that ACCF/AHA guidelines, which recommend patients with new or presumed-new LBBB undergo early reperfusion therapy (fibrinolysis or PCI), are based on studies from more than 20 years ago. Citing newer research, the authors observe that a substantial proportion of patients who present with LBBB are not actually experiencing a STEMI-equivalent MI: For example, in 2007 Larson and colleagues reported that among patients with LBBB at presentation, the rate of false activation of the catheterization laboratory was 44%, compared with a baseline of 14%. For patients with presumed-new LBBB and suspected MI, Neeland and coauthors propose a new algorithm involving Sgarbossa criteria on ECG, bedside echocardiography, and serial echocardiogram, as shown here.
The Interview
Ryan: Do you often get called by the ED or house staff about a “new” LBBB?
McEvoy: Yes, this is a fairly common clinical dilemma at our institution. Previous registry studies would suggest that only about 2% of acute coronary syndrome patients present with LBBB. However, such studies did not include patients who were catheterized for LBBB but did not actually have ACS. Indeed, up to 40% of patients who undergo cardiac catheterization for LBBB are not found to have occlusive coronary artery disease. This has been my personal experience as well. Therefore, some catheterization-lab activations may be unnecessarily triggered by new LBBB and “concern for ACS.” Anecdotally, at least as many of these calls come from our general inpatient floors as from our ED. That’s presumably because such patients are often transferred from outside hospitals or directly admitted without prior EKGs recorded in our electronic medical record.
Neeland & de Lemos:We agree with Dr. McEvoy. Only a small minority of patients presenting with a suspected ACS have LBBB, but this group is over-represented among patients who prompt a false activation of the catheterization lab. In a recent study by McCabe et al., of the 146 patients with false STEMI lab activations, over 10% had LBBB. Notably, these figures reflect only patients who make it all the way to cardiac catheterization. In our institution and probably many others, it’s not uncommon to “deactivate” for a patient with LBBB whose clinical scenario is not consistent with STEMI.
Ryan: What do you normally do? And how has your practice evolved from when you were a resident, to junior fellow, and now a more-senior fellow at Hopkins?
McEvoy: My threshold to act on any suspicion for ACS has been—and remains—very low. However, my approach has become more nuanced. As a resident, I learned that although “time is muscle,” one needs to “treat the patient and not the EKG.” Of course, the EKG is more than enough to proceed to catheterization if the patient is unstable or has elevated cardiac enzymes. However, we are not currently considering such a straightforward scenario.
In cases (not uncommon) where the story is atypical, the vital signs and exam are stable, and the enzymes are initially negative, my approach has changed. I have found it helpful to get aggressive about chasing down old ECG reports in such cases. Primary care providers and outside hospital records can often establish that the LBBB is old, even when the actual ECGs are not obtained. If the LBBB is old, the management can change drastically. Similarly, if the LBBB is definitively new, I think the threshold to perform cardiac catheterization should be extremely low. However, a management dilemma often arises when the LBBB is not proven to be old or new in this type of intermediate-risk patient. I have come to see in these cases that the ECG, on its own, is not a helpful bedside triage test.
Thankfully, these are the very cases when you may have some time to consider everything before activating the catheterization lab. Of course, such a strategy may jeopardize the door-to-needle or door-to-balloon time if the presentation is, in fact, a real ACS. If such a patient presents very early, it is often interesting to obtain a STAT myoglobin level (although I suspect such a maneuver will be superseded by highly sensitive troponin assays and circulation endothelial cells). However, my usual strategy is to bring an echocardiography machine directly to the bedside. We know that ACS-induced LBBB is caused by a large anterior infarct. Therefore, a normal echocardiogram would reassure me in the right clinical scenario.
As a fellow, I also like to discuss all intermediate-risk patients who present with possible-new LBBB with the on-call interventionalist. It is important to acquire as much information as possible (including echocardiographic data) within the required time window, to facilitate a complete discussion of the case with interventionalist colleagues.
Neeland & de Lemos: We agree that the decision threshold to activate the catheterization lab should be low, given the clear benefits of timely PCI and the relatively less important consequences of false cath-lab activation versus lytic administration. It may also differ a bit depending on whether the cath-lab personnel are present or must be called in from home.
At our institution, we’ve created a rapid fellow/faculty consult pathway for ED cases where STEMI is ambiguous or the indications for emergent cath are not clear. This represents a “stop and take a breath” approach for cases where primary PCI is not obviously indicated. LBBB patients make up a sizable proportion of this pathway. Bedside echocardiography, a class IIa indication in this setting, can be very helpful to differentiate an acute ischemic syndrome from chronic disease in a patient with LBBB.
We think it’s important to emphasize that even “new” LBBB may not necessarily translate to an occluded coronary artery/STEMI equivalent. In our experience, truly new LBBB from complete coronary artery occlusion is very rare, given the size and extent of infarcted myocardium necessary to cause a new LBBB; so most of the cases of even new LBBB are not concordant with an occluded coronary artery. In fact, from 4 contemporary angiographic studies of patients presenting with ACS and LBBB, the prevalence of a STEMI-equivalent MI ranged only from 7% to 61% among those with a new LBBB. In Dr. de Lemos’s experience, the prevalence is much closer to 7% than 61%! We’ve seen very few true acute LBBB “STEMI equivalents” in our entire careers. We’d be very interested to hear from other experienced cardiologists about how often they see “the big one” in the LBBB population in contemporary practice.
Ryan: Some people argue that it’s better to err on the side of caution by taking the “new” LBBB to the lab. Do you agree?
McEvoy: We should never allow a truly ischemic LBBB to go without rapid revascularization. Again, I have a very high threshold to not catheterize a proven-new LBBB. So I tend to agree with the above sentiment. However, such a strategy may be somewhat outdated in the modern era, particularly in those “grey zone” cases when the previous EKG is not available and the LBBB cannot be proven as either old or new. Specifically, we now have additional diagnostic tests, complementary to the EKG, which can be brought to bear in a timely manner. Echocardiography can be rapidly performed in most institutions (usually on a 24-hour basis). Whether CT coronary angiography will also find a role in the work-up of such cases is another interesting research question. I do realize that this may not be realistic in all medical facilities. However, any such patient presenting to a facility with limited resources must be transferred to a facility with a catheterization lab. Those facilities also have the ability to do an echocardiogram on arrival.
Neeland & de Lemos: It’s easy for those of us who don’t take interventional calls to say we should have a very low threshold! However, these false activations do put patients at some risk — and are demoralizing for the interventionalists and cath lab staff. That said, we agree that the downside risks of delayed reperfusion and missed diagnoses may matter even more. The challenge is to weigh these opposing risks and make the right decision for the patient. Additional information — ECG, biomarker, or echo-based — can be helpful in these situations, which is where our proposed algorithm should be applied.
Ryan: Neeland and colleagues suggest that because patients with LBBB have an overall higher risk for bleeding, they should, if possible, be preferentially transferred for primary PCI. In areas without cath lab access, what are your thoughts on fibrinolysis in patients with presumed new LBBB? And what was your approach to this issue in Ireland, where you worked as a cardiology registrar/fellow before coming to the U.S.?
McEvoy: For me, the fundamental question is not one of geography, but simply whether a patient presenting with LBBB needs to be rapidly considered for revascularization or not. Again, if the patient presents with clearly new LBBB, a good ACS story, or positive enzymes, revascularization must be prompt. However, if the LBBB duration is unknown, the story is atypical, and the enzymes are negative, a decision about the benefit of rapid triage for revascularization still needs to be made. If such a patient is deemed likely to benefit from revascularization (possibly with echocardiographic guidance) and the door-in/door-out (DIDO) time prior to transfer is <30 minutes (or the difference in door-to-needle time versus door-to-balloon time is <60minutes), the patient should be transferred for primary PCI. In addition to the bleeding risk with thrombolysis in LBBB, I would also suspect that the anterior and massive distribution of ischemia required to cause LBBB inherently means that the patient would derive probabilistic benefit from PCI. Unfortunately, in Ireland these temporal targets were often not realistic and thrombolysis would need to be performed.
Neeland & de Lemos: Given that the risks of bleeding are higher in patients with LBBB (because of a greater likelihood of comorbidities) and the chance of an occluded artery is lower, fibrinolysis is a riskier endeavor in these situations. Data from Pinto et al. suggest that longer PCI-related delay (up to about 2 hours) may still have a favorable risk/benefit ratio than fibrinolytic therapy. In older patients, such as those who present with LBBB, the risk/benefit tradeoff may occur even later. We feel that LBBB patients should be preferentially transferred to centers capable of primary PCI for these reasons and that fibrinolysis should be discouraged unless PCI really cannot be performed within a reasonable time frame. Another issue is that positive enzymes in the absence of a suggestive clinical history, even in the setting of a new LBBB, should not automatically be considered an ACS. Chronic elevations in troponin are common in these patients, related to concomitant structural heart and renal disease, and the trend of the enzymes can be very helpful to differentiate ACS from non-ACS causes of elevations.
Ryan: What do you think of this new proposed algorithm?
McEvoy: My biggest concern about this well-thought-out algorithm is the complete exclusion of the clinical history. I think a convincing and concerning history for ACS in the setting of LBBB should prompt rapid consideration for revascularization. I worry that not including the history and also the pretest probability for ACS (Diamond and Forrester, Duke clinical score, Genders score, etc.) in the algorithm may potentially lead to delays in unstable patients while echocardiography or serial enzymes are being performed. Multiple studies have also now demonstrated the value of CT coronary angiography in triaging patients presenting to the ED with intermediate probability of ACS. Interestingly, recent research also suggests that coronary artery calcium testing (with noncontrast CT) may also be useful in this situation. I would suggest that such tests could also be effectively incorporated into this algorithm in many cases.
Neeland & de Lemos: We completely agree with Dr. McEvoy’s point that the clinical history is a cornerstone of correct diagnosis and management. The first box of our proposed algorithm begins with LBBB and a clinical scenario consistent with ACS (“suspected ACS”), and it should not be extrapolated to situations where the pretest probability of ACS is low. The points about CT angiography are interesting, but it may be challenging to use in patients with LBBB, given their usually older age and greater probability of coronary calcification (which can create technical challenges), as well as a higher likelihood of diffuse coronary disease. We hope that our Viewpoint article will prompt more research into new approaches for rapid differential diagnosis of the patient with LBBB and possible ACS, because we are not satisfied with our current diagnostic and management approach.
Share your views on the new algorithm proposed by Neeland and colleagues.

I am curious to know if the authors have seen the work of Smith et. al from Hennepin County Medical Center that considers a modified form of Sgarbossa’s least specific criterion (discordant ST-elevation = or > 5 mm). In place of this criterion they consider the ST/QRS ratio with a value of 0.2 indicating acute STEMI (excessive discordance) which has been found to be both more sensitive and more specific. As a rule of thumb I allow 1 mm of ST-elevation (measured at the J-point) for every 5 mm of S-wave depth.
Yes, we’re definitely aware of the work of Smith and Dodd in this area and we reference their research letter in our paper. The reference is:
Smith SW, Dodd KW. Outcomes in patients with chronicity of left
bundle-branch block with possible acute myocardial infarction [letter].
Am Heart J 2011;162:e23.
They report that “excessive discordance” on ECG, defined as a ratio of ST-segment elevation to S-wave amplitude (depth) of -0.20 or less to be 84% sensitive and 99% specific for left anterior descending coronary artery occlusion in a study of 148 patients with LBBB and suspected AMI. The reasons for the “negative” sign is that the ST-elevation and the S-wave amplitude point in opposite directions.
This additional criterion is promising but I haven’t seen their work published outside of this research letter. It’d be great to have more data on this criterion and to see their findings reproduced in another cohort of LBBB patients.
As a frontline ED physician and a STEMI systems activist, I am also very concerned that “new LBBB” is a poor predictor of appropriate Cath Lab activation….unless there is a truly compelling clinical history.
I certainly hope the 2012 STEMI GL demote “new LBBB” and Core Measures do not rely on its inclusion.
Multiple papers demonstrate the poor utility of New LBBB….including two papers that I lead with a multi-disciplinary group ( AHJ 2010 review and a HORIZONS sub-analysis in Cath CV Intv 2012), as well as Kontos AHJ.11 , Jain and Ting from Mayo AJC.11, Baran from St Paul in Circulation Qual.10, and Mixon and Dehmer from Texas in Circ Qual.12
Great discussion,
In the suggested algorithm Figure, you separate out “masked STEMI” and “NSTEMI/UA” which is practically impossible to distinguish by ECG in the setting of LBBB as you correctly elude in the article.
I was wondering what your thoughts are about modifying the algorithm downstream of “Bedside Echo/Serial biomarkers” into a more distinct set of criteria such as “New Wall motion abnormality or rapid elevation in cardiac biomarkers” that would help us identify which patients to Reperfuse Emergently. I think this group of patients (hemodynamically stable, no sgargbossa concordance) is the most controversial and future research delineating more concise Echo/biomarker criteria would be of great benefit in this dilemma.
A concern I have regarding ‘new wall motion abnormality’ is that the echo can often demonstrate a wall motion abnormality and dyskinesia from just LBBB alone. This is another limitation with relying soley on ECHO in this situation which I did not discuss originally. In my experience, the means of echo acquisition (out of hours, bedside, multiple distractions, etc) in these patients also limits the interpretation of many STAT ECHOs. I do agree that future research is required in this area, but want to stress the importance of putting all of the pieces of the clinical puzzle together, rather than just relying on any one piece alone.
I totally agree Dr. McEvoy,
That’s why I raised the question in the first place. I believe that It is very difficult to use Echo and serial biomarkers to differentiate “Masked STEMI” from “NSTEMI/UA” as it’s shown in the algorithm and I don’t think we can make decisions about emergent reperfusion in this group of patients (sgarbossa concordance negative, hemodynamically stable) accurately with the suggested strategy. I agree with stressing the importance of putting the whole picture together and the need for future research to stratify this controversial group of patients.
I agree with Dr. McEvoy that one needs to “put all of the pieces of the clinical puzzle together” when evaluating a patient, especially one in whom the diagnosis is difficult to ascertain. Use of echo to clarify the diagnosis of STEMI in the setting of LBBB however is a Class IIa recommendation from the ACCF/AHA guidelines and can be used effectively in many cases. Although one can often see abnormal septal motion on the echo due to LBBB, the presence of a large anterior wall motion abnormality, especially one that was not present on prior studies and if there are no other telling signs of chronic systolic dysfunction or structural heart disease, is not common in LBBB and should signal possible STEMI or large territory NSTEMI and prompt consideration of urgent revascularization.
Dr. Elshalzy correctly points out the difficulty in differentiating STEMI from NSTEMI when the ECG is not specific for STEMI. Prospective study is definitely needed before the strategy of rapid biomarker testing is employed, but some new data recently published in Circulation shows some promise in using the absolute change in hs-cTnT after only 1 hour from initial testing to differentiate chest pain due to CAD vs. non-cardiac causes. Given that LBBB patients often have baseline elevation in hs-cTnT due to other cardiac disease, perhaps rapid testing for absolute changes would help to distinguish those in whom we should consider for more rapid reperfusion therapy.
I do agree with the algorithm suggested. In our practice, when we get an ECG with LBBB, first we ask about the presentation and any past ECG available. If it is proved to be newonset LBBB with typical chest pain, we proceed for PCI. If the history is not suggestive of AMI, we go for next step with echocardiography and biomarkers. If the reports are suggestive of AMI, PCI will be the line of management, if not further work up regarding other possible diagnosis. In my practice, I have to still see cases with new onset LBBB without chest pain, having total occlusion in proximal LAD. New onset LBBB with chest pain and angiography showing total occlusion in proximal LAD not an unusual scenario.
I have two questions for the authors.
1/ The proposed algorithm is referring patients with heart failure directly to emergent reperfusion, yet a lot of patients with LBBB may present not only with de nove decompensation but also with acutely decompensated chronic heart failure where LBBB is the sign of long-standing structural heart disease. Are there any data that patients with HF plus LBBB have frequent occlusive arterial disease? What are angiography findings in patients they mention, i.e. those with „atypical chest pain or evidence of new onset heart failure in whom LBBB is present but cannot be confirmed to be old.“
2/ Do the authors see false diagnoses of LBBB as the reason for false cath-lab activation? (I refer to the following paper: Defining Left Bundle Branch Block … by Strauss et al. in: Am J Cardiol 2011;107:927–934; the paper has the value not only in regard to CRT indication, but to correct diagnosis of LBBB as such.
Conventional criteria for LBBB now in use lead to false diagnosis of complete LBBB in about a third of patients, while QRS morphology is crucial for the right diagnosis.
(I fully agree that alarming clinical scenario with corresponding history is a clear indication for early PCI irrespective of LBBB duration.)
Great questions, Dr. Kostek. In reference to your first question, I am not aware of any specific studies looking at the relative frequency of occlusive disease in patients with LBBB and HF. Just by the probability though, these patients tend to be older, many with ischemic cardiomyopathy, and therefore have a greater likelihood of significant lesions. In the paper, when we referred to the not uncommon scenario of cath lab activation for patients with heart failure and LBBB, it was to confirm anectodally that fasle positive cath lab activation is not infrequent in this setting. Nevertheless, as you point out, it would be difficult not to take someone to the cath lab when they are in hemodynamic extremis, which is why we have those patients going directly to reperfusion in the algorithm. Again though, it should be in the proper clinical setting of suspected ACS and not simply acute decompensated HF without any other clinical suggestion of AMI.
In reference to your second question, thank you for that interesting reference, I had not seen that article and it definitely is pertinent to the discussion. Personally, I have not seen a case of cath lab activation for new LBBB when the LBBB is not really a LBBB but rather a LAFB with LVH (as those authors describe). I will defnitely take a closer look though after reading that article!
Thanks for the answers and one more comment/question. Angiography may be rewarding in case of finding the culprit artery whereas intervening some “oculostenotic” lesions in pts with LBBB plus HF may not prove beneficial. If you find some stenoses what is your next step (FFR measurement or any other)?
When patients with new LBBB and HF with suspected STEMI-equivalent MI are referred for urgent angiography and an occluded culprit IRA is found, my practice preference would be to intervene only on the culprit IRA at that time and not on any other lesions, even if they are visually “significant,” since the risk of adverse outcomes increases when intervening on non-culprit artery lesions in the STEMI setting.
Here comes the role of FFR in multivessel stenting, .In STEMI with cardiogenic shock multivessel strategy is preferred.