April 8th, 2011
Who Might Merit the MitraClip?
Ted Feldman, MD
CardioExchange welcomes Ted Feldman, lead investigator for the EVEREST II study published earlier this week in the NEJM and presented at the ACC Scientific Sessions in New Orleans. Drs. Richard A. Lange and L. David Hillis, of CardioExchange, asked Dr. Feldman about the nuances of this randomized trial, in which percutaneous repair was compared with surgery in patients who had mitral regurgitation. We encourage you to offer your own questions and opinions.
Background: In EVEREST II, 279 patients with moderately severe or severe mitral regurgitation (MR) were randomized in a 2:1 ratio to undergo either conventional surgery (valve repair or replacement) or percutaneous repair with the MitraClip, an experimental device. The device maker funded the trial.
Rates for selected trial endpoints appear in the table below.
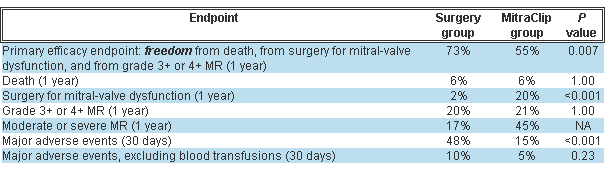 Post-treatment MR severity improved in both groups but significantly more in the surgery group. About a quarter of the MitraClip group had significant MR prior to hospital discharge and were referred for surgery.
Post-treatment MR severity improved in both groups but significantly more in the surgery group. About a quarter of the MitraClip group had significant MR prior to hospital discharge and were referred for surgery.
Drs. Lange and Hillis: Before everyone and his dog signs up for training, tell us which MR patients are good candidates for percutaneous repair, and who should not undergo the procedure.
Dr. Feldman: Higher-risk patients are the clearest candidates for the MitraClip. A subgroup analysis from the trial points to the best outcomes in patients age 70 or older, those with LV ejection fractions <60%, and those with functional MR. We have substantial experience showing excellent clinical results in patients who are high risk for conventional mitral valve (MV) surgery, with safety outcomes equivalent to those in the randomized trial. Compared with a matched control group of high-risk registry patients (mean Society of Thoracic Surgeons [STS] risk score >12%), MitraClip recipients have a 45% lower rate of hospitalization during the year after therapy, and significantly better survival. Most of these patients have functional MR, and we find results to be the same for functional versus degenerative MR. Decisions about therapy for MR have always been complex and patient-specific; the MitraClip adds an option for many patients even if it also adds complexity. Of course, suitable valve anatomy is essential (the MR must arise from the A2–P2 scallops). For flail leaflets, the flail gap must be <10 mm and the flail width <15 mm.
Lange and Hillis: The procedure sounds like a real tour de force (i.e., general anesthesia, transthoracic echo, multiple interventionalists, and so on). How long does the procedure take, and how many physicians are involved?
Feldman: The mean procedure time now is between 1 and 2 hours. Single-clip cases often take less than 1 hour. Two-clip cases, which constitute 40% of the experience to date, typically take longer and are ordinarily closer to 2-hour procedures. In our earlier experience procedures took longer, and certainly the first few procedures for new operators each take several hours because the learning curve is substantial. The procedure is performed under general anesthesia with transesophageal echocardiographic guidance, so typically there are 2 interventional physicians, an echocardiographer, and an anesthesia team in the cath lab.
Lange and Hillis: Compared with conventional surgery, percutaneous treatment was associated with a higher 1-year incidence of subsequent surgery for MV dysfunction and of moderate or severe MR. Given that improvements in MR severity and LV end-diastolic volumes were greater with MV surgery than with percutaneous repair, why should patients be referred for percutaneous treatment?
Feldman: Of the MitraClip recipients in the trial, 78% did not require surgery after 2 years. Also at 2 years, the percentage of patients in NYHA class 1–2 is higher for the MitraClip group than for the surgery group (99% vs. 88%; P<0.05). Among those who have inadequate control of MR, surgery (including repair) remains an option. When the strategy of MitraClip with surgery (if needed) is compared with surgery as a first therapy, the two approaches show no difference in efficacy outcomes at 2 years. Patients prefer less invasive therapy, as reflected in the fact that 16% of the group randomized to surgery ultimately didn’t have surgery. The repair rate in EVEREST II is substantially higher than that reported in the STS database, but one must also consider that some patients referred for surgery undergo mitral replacement rather than repair.
Lange and Hillis: Patients with residual severe MR after percutaneous repair who successfully underwent MV surgery were counted as having had a successful outcome. Most clinicians would consider the outcome “clinically successful” when device deployment is accomplished without a major complication (death, MI, or cerebrovascular accident) and follow-up reveals no or only mild MR. By this definition, how often is percutaneous repair of MR clinically successful?
Feldman: Adverse safety events were less common with the MitraClip than with surgery, and major complications such as death, CVA, and need for urgent surgery occurred in MitraClip patients almost exclusively when they had later elective surgery. We examined several measures of clinical success. Surgery resulted in greater reductions in MR. Both groups had improved LV-chamber dimensions at 1 and 2 years, and NYHA class was better for MitraClip patients at both junctures. Not needing surgery (almost 80% of cases), feeling well, and improved LV function at 2 years may reflect “clinical success” from a patient perspective.
Lange and Hillis: The safety of percutaneous treatment is touted as superior to that of MV surgery, but transfusions were the largest single component of the major adverse events in EVEREST II. After transfusions were excluded, the rate of adverse events was statistically similar in the two groups. Did transfusions have an important effect on late outcomes?
Feldman: The impact of transfusions on outcomes has been the subject of much discussion. Numerous studies in the surgical literature demonstrate a clear acute and late effect of transfusions on mortality, compared with mortality in nontransfused cardiac surgery patients (e.g., Circulation 2007; 116:2544). The mortality risk has been shown to correlate with the number of units of transfused blood. Both adjusted and unadjusted analyses reveal a relative risk for mortality of at least 1.7 for transfused patients after cardiac surgery. I think it is fair to conclude, as we have seen after PCI, that transfusions contribute to adverse outcomes after cardiac surgery.
Lange and Hillis: Of the surgical group, 86% underwent MV repair. Of patients in the percutaneous-repair group who had severe residual MR and were referred for surgery, nearly half underwent MV replacement. Does percutaneous repair affect the patient’s eligibility for MV repair? How about after 3 or 4 years have passed?
Feldman: Surgical repair has been accomplished successfully after MitraClip therapy as late as 5 years after clip implantation. In a multivariate analysis of data from EVEREST II — presented by our surgical PI, Don Glower, at TCT — complex leaflet pathology (bileaflet/anterior flail) independently predicted the need for MV replacement, regardless of whether surgery was the first therapy or was performed after MitraClip implantation. Surgeon experience and duration of implant did not appear to affect the repair/replacement rate. Nor did the repair rate differ between patients who had an operative note of valve injury or difficulty removing the device and patients who did not have the operative note. Our experience has shown that MV surgery can be performed safely following the MitraClip procedure, with results similar to the control group at both 30 days and 1 year.

A big advance…but major caveats. As I mentioned in a previous post, the relative equivalence between the two arms in the functional group is likely a reflection on poor surgical approaches to this predominantly ventricular problem. Most asymmetric ischemic functional MR is from A3-P3 retraction (with some middle scallop involvement). I have still qualified such patients for the procedure.
The transfusion issue may be overblown, as the harm is mostly shown in retrospective trials and mostly CABG patients (not isolated mitrals). Also most of the harm occurred within 2 years.
The concern is reflected by a case I heard about while training for 3D echo at a high volume mitral clipping center: a 55 yo with severe MR from P2 prolapse referred for the clip. Hard to justify for a good surgical candidate. Also, I do find the primary surgical endpoint low for the degenerative subgroup… my repair expectations are about 95% in experienced hands (no matter which segments are involved).
Competing interests pertaining specifically to this post, comment, or both:
None