December 17th, 2012
New Guidelines Define State-of-the-Art STEMI Care
Larry Husten, PHD
New guidelines published online today in Circulation and the Journal of the American College of Cardiology provide an efficient overview of the best treatments for STEMI patients. (Available for download are PDFs of the full version [64 pages] or the executive summary [27 pages] of the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction.)
“We’re looking to a future where more patients survive with less heart damage and function well for years thereafter,” said Patrick O’Gara, the chair of the guidelines writing committee, in a press release. “We hope the guidelines will clarify best practices for healthcare providers across the continuum of care of STEMI patients.”
The new document strongly supports the establishment and maintenance of regional systems to treat STEMI, which should include assessment and continuous quality improvement programs.
Primary PCI remains the preferred method of reperfusion when it can be performed by experienced operators in a timely fashion. For people who can’t receive primary PCI within 120 minutes of arrival, fibrinolytic therapy should be given within 12 hours of the the onset of symptoms.
The first medical contact (FMC)-to-device time should be 90 minutes at PCI-capable hospitals. Patients who arrive at non PCI-capable hospitals should be transported to a PCI-capable hospital within 30 minutes and should be treated with a FMC-to-device system goal of 120 minutes or less.
Drug-eluting stents should not be used in patients who can’t or won’t comply with long-term dual antiplatelet therapy (DAPT). After receiving a stent, patients should receive DAPT with aspirin and either clopidogrel, prasugrel, or ticagrelor.
December 16th, 2012
A Compelling Cardiac Surgery Broadcast — in Norway
Øystein Horgmo, BSc
This material has been imported, with permission, from The Sterile Eye.
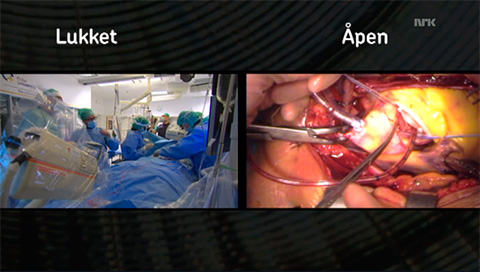
- Screenshot from nrk.no.
Last week in Norway, surgical and percutaneous aortic valve replacements were broadcast in their entirety on television. This is the full-length feature the Norwegian Broadcasting Corporation (NRK) offered their viewers last week. And it turned out to be a night of public education on the complexities of surgery.

- The “closed” minimally invasive procedure. Screenshot from nrk.no.
The two aortic valve replacements were both done at Oslo University Hospital. The traditional open surgery and the minimally invasive TAVR (“closed”) procedure had been recorded in their entirety by the NRK. In the broadcast, they were shown side by side with the focus alternating between them at different key points.

- Studio discussion. Screenshot from nrk.no.
NRK’s stroke of genius was to have a live running commentary, or rather a conversation, in the studio between the hostess and a senior cardiac surgeon. That was a much better solution for the wide viewing audience, rather than having the surgeons comment as they were operating. The hostess asked questions most lay people would wonder about, such as “Doesn’t the guidewire hurt the blood vessels?” and “What was it like to cut through a sternum for the first time?” The surgeon in the studio answered in a nonacademic way, not afraid to address problems or difficult subjects.
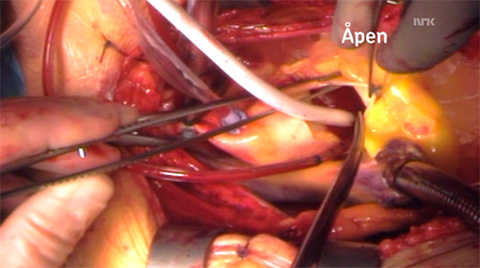
- The open procedure. Screenshot from nrk.no.
An interesting thing, which I didn’t see coming, was that the program explored the pros and cons of both minimally invasive and open procedures. My feeling is that the general public tends to view minimally invasive procedures as “better” and safer than open procedures. However, the surgeons had great difficulty placing the guidewires and pacemaker in the percutaneous procedure, whereas during open surgery the work was swift and without delay. The advantage of the open field was quite clear to anyone who watched. It’s a function of the two patients who were chosen, of course, but nonetheless a good illustration of the complexities involved in choosing one procedure versus the other.
The program is in Norwegian, but for those of you who would like to watch it anyway, check out the links to some of the key moments in the two operations below — or watch the entire show here.
December 14th, 2012
CHMP Recommends Against Approval for Mipomersen in Europe
Larry Husten, PHD
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency today recommended that mipomersen (Kynamro; Isis and Genzyme) not be approved for use in Europe. The novel antisense oligonucleotide works by inhibiting the synthesis of apolipoprotein-B and is under development in the United States and Europe for the treatment of familial hypercholesterolemia.
CHMP agreed that mipomersen effectively lowered LDL cholesterol in people with homozygous and severe heterozygous familial hypercholesterolemia (FH). The negative recommendation was based on the committee’s concerns about the safety of mipomersen. Here is the CHMP discussion of the safety issue:
The CHMP was concerned about the medicine’s safety. The Committee noted that a high proportion of patients stopped taking the medicine within two years, even in the restricted group of patients with homozygous familial hypercholesterolemia, mainly due to side effects such as flu-like symptoms, injections site reactions and liver toxicity. This was considered important because Kynamro is intended for long-term treatment in order to maintain the cholesterol-lowering effect. The CHMP was also concerned by liver test results in patients taking Kynamro showing a build-up of fat in the liver and increased enzyme levels, and was not convinced that the company had proposed sufficient measures to prevent the risk of irreversible liver damage. Moreover, the Committee was concerned that a greater proportion of patients taking Kynamro experienced serious cardiovascular events (problems with the heart and blood vessels) than patients taking placebo. This prevented the CHMP from concluding that Kynamro’s intended cardiovascular benefit, in terms of reducing cholesterol levels, outweighed its cardiovascular risk.
In October, the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee gave a weak endorsement to mipomersen, voting 9-6 in favor of an approval recommendation. Comments during the panel meeting, however, clearly indicated that committee members were concerned about both the efficacy and safety of the drug.
Another new drug under development for lowering cholesterol in FH patients is lomitapide (Aegerion). The same FDA panel in October gave lomitapide a slightly stronger approval recommendation.
December 13th, 2012
Hypertension And Smoking Top List Of Global Risk Factors
Larry Husten, PHD
Worldwide, hypertension and tobacco smoking are the single largest causes of death and disability, according to findings from the Global Burden of Disease Study 2010 (GBD 2010), the largest ever assessment and analysis of global health and disease. In an unprecedented move, the Lancet devoted an entire issue to the study, including seven separate articles and eight comments.

GBD 2010 was led by the Institute for Health Metrics and Evaluation (IHME) at the University of Washington. In a press release, IHME director Chris Murray said, “For decision-makers, health-sector leaders, researchers, and informed citizens, the global burden of disease approach provides an opportunity to see the big picture, to compare diseases, injuries, and risk factors, and to understand in a given place, time, and age-sex group, what are the most important contributors to health loss.”
Despite significant reductions in the rate of ischemic heart disease and stroke since 1990, overall these retained their position as the #1 and #2 worldwide causes of death. Among men 15-49 years of age, CV disease was the single largest cause of death, accounting for 12.8% of all deaths. For women of the same age CV disease was the third largest cause of death, following HIV/AIDS and other non-communicable diseases, accounting for 10.7% of all deaths.
Ischemic heart disease in 2010 now ranks as the largest single cause of global years of life lost. In 1990 it had ranked fourth, behind lower respiratory infections, diarrhea, and preterm birth complications. Stroke moved from fifth place to third place.
High blood pressure emerged as the single most important risk factor for death and disability, followed by tobacco smoking. In 1990 the top two risk factors were childhood underweight (#8 in 2010) and household pollution (#4 in 2010).
December 13th, 2012
Diabetics with Multivessel Disease: FREEDOM with CABG?
Valentin Fuster, MD
FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease), a large NIH-sponsored study presented at AHA 2012 and published simultaneously in the New England Journal of Medicine, showed that diabetics with multivessel disease had lower rates of death and MI with CABG than with PCI, but a higher rate of stroke. Study author Dr. Valentin Fuster answered CardioExchange’s questions about FREEDOM and what it means for patients.
Click here to listen to the original audio interview.
Q: What will you tell your patients with diabetes who would have met inclusion criteria for FREEDOM? How will you help them to understand their options?
A: The problem is that most patients with diabetes who undergo cardiac catheterization already have two- or three-vessel disease. We have to tell the patients prior to the catheterization that the angiogram will probably show disease for which surgical intervention is the option of choice. This is a different approach from what we’ve been doing, which is when catheterization is often immediately followed by a decision to do an angioplasty or insert a stent. Patients need to be informed of their options beforehand.
Q: So the point here is to prevent ad hoc PCI?
A: Yes, it’s beyond whether a patient is a candidate or not. It usually begins in the cath lab. The main reason that people were rejected from our study was because they indicated at the time of the procedure that they would prefer an angioplasty over surgery, which ruled them out as a participant.
Q: What was the nature of the strokes in FREEDOM?
A: Of the patients who had them, 60% of those in the PCI group and 70% of those in the CABG group experienced some degree of disability , and 27% of those in the PCI group and 55% of those in the CABG group experienced a significant degree of disability. The number of strokes was relatively small and they occurred throughout the study, rather than only at the time of the procedure. We actually believe that there were fewer strokes in the PCI group over the long term because they were taking clopidogrel and aspirin together, whereas the majority of the CABG group were taking aspirin alone. We are planning to test this hypothesis.
Q: Was there any heterogeneity in outcome by the diabetes severity?
A: Most of the patients had type-2 diabetes, and the average hemoglobin A1c level was 7.8%, however, there was a large group that had more than 7% and another group that had less than 7%. Regardless of the degree of diabetes control, the results were equal in favor of CABG.
Q: Do you think the results are relevant to patients in their 80s — for whom CABG might be a bigger deal?
A: There were very few patients who were age 80 or older. I would say that at that advanced age the concern of survivorship, myocardial infarction, and stroke are secondary compared with whether or not you can relieve the patient’s angina. For a patient at that age, I would perhaps give a second thought to a stent for specific individuals after full discussion. I’m not necessarily in favor of stenting, but, depending on what your goal is, it should be discussed. If the goal is to relieve angina that might be accomplished with simple stenting.
December 12th, 2012
FDA: Small, Nonsignificant Risk from Chantix
Larry Husten, PHD
The FDA today updated its safety review of the smoking cessation drug varenicline (Chantix, Pfizer). A large meta-analysis, which the FDA had required Pfizer to perform, found a higher rate of major adverse cardiovascular events (MACE) in patients taking varenicline than in patients taking placebo. However, the increase in risk was very small and did not achieve statistical significance. The FDA concluded that “it is uncertain whether the excess risk for the Chantix group was due to the drug or due to chance.”
The FDA said the results of the meta-analysis are consistent with findings of an earlier trial described in a previous FDA communication. The new meta-analysis utilizes data from 7002 patients who were randomized to placebo or varenicline in one of 15 double-blind trials.
The FDA reported a low MACE rate for both groups. Although varenicline-treated patients had nearly double the risk for an event as the placebo recipients, the confidence interval was wide. The FDA noted that cardiovascular mortality and all-cause mortality was slightly lower in the varenicline-treated group, though the difference was of course not statistically significant.
Here are the data from the meta-analysis:
MACE: varenicline 0.31% [13/4190] vs. placebo 0.21% [6/2812]
- Adjusted hazard ratio: 1.95 (95% CI, 0.79-4.82)
Cardiovascular mortality: varenicline 0.05% [2/4190] vs. placebo 0.07% [2/2812]; P=ns
All-cause mortality: varenicline 0.14% [6/4190] vs. placebo 0.25% [7/2812]; P=ns
December 12th, 2012
State of the Heart: AHA Publishes Year-End Statistical Update
Larry Husten, PHD
Although deaths from cardiovascular disease have been declining for many years, continued progress is threatened by disturbing trends in U.S. lifestyles. That’s the clear message from the American Heart Association’s year-end report, “Heart Disease and Stroke Statistical Update 2013,” published in Circulation. “Americans need to move a lot more, eat healthier and less, and manage risk factors as soon as they develop,” said Dr. Alan S. Go, the chairman of the report’s writing committee, in an AHA press release. “If not, we’ll quickly lose the momentum we’ve gained in reducing heart attack and stroke rates and improving survival over the last few decades.” Here are some of the key statistics contained in the hefty report: “The Epidemic of Poor Health Behaviors.”
- Among adults, 21.2% of men and 17.5% of women continue to smoke cigarettes. 18.1% of high school students are smokers.
- Among high school students, 17.7% of girls and 10.0% of boys report they did less than one hour of moderate-to-vigorous exercise per week.
- Thirty-three percent of adults reported engaging in no aerobic leisure-time physical activity.
- From 1971 to 2004, calorie intake increased from 1542 to 1886 kcal/day (22%) in women and from 2450 to 2693 kcal/day (10%) in men. Most of the change is due to an increased consumtpion of starches, refined grains, and sugars.
- 68.2% of adults are overweight or obese; 34.6% are obese.
- 31.8% of children 2 to 19 years of age are overweight or obese; 16.9% are obese.
“Prevalence and Control of Health Factors and Risks Remains an Issue for Many Americans”
- 13.8% of U.S. adults have serum serum cholesterol levels ≥240 mg/dL.
- 33.0% of U.S. adults have hypertension. About 82% are aware of their condition, and 75% receive antihypertensive therapy, but only a little more than half (53%) have achieved target blood pressure levels.
- 8.3% of U.S. adults have been diagnosed with diabetes; 38.2% have abnormal fasting glucose levels (prediabetes).
CV Disease and Mortality
- Although the percentage of deaths attributable to CV disease has been declining for decades, in 2009 CV disease was responsible for nearly one-third (32.3%) of all deaths in the U.S.
- About 635,000 people have a first MI or CHD death each year. About 280,000 have a second MI.
- About 795,000 people have a new or recurrent stroke each year.
- The 2009 total direct and indirect estimated cost of CVD and stroke was $312.6 billion.
- The 2008 total direct and indirect estimated cost of all cancer and benign neoplasms was $228 billion.
December 12th, 2012
Boehringer Ends Phase 2 Trial of Dabigatran in Mechanical Valve Patients
Larry Husten, PHD
Boehringer Ingelheim today announced that it had discontinued a phase 2 trial of its anticoagulant drug dabigatran (Pradaxa) in patients with mechanical heart valves. As reported here in October, the company had previously terminated one arm of the study after an interim review of the data by the trial’s Data Safety Monitoring Board.
The RE-ALIGN trial was an open-label, 12-week randomized comparison of warfarin and dabigatran in 400 patients who received a mechanical valve. The first arm randomized patients during their initial hospital stay; the second arm randomized patients more than 3 months after their surgery.
Despite the recent advent of novel oral anticoagulants, the much-maligned warfarin remains the only current option available for patients who have received a mechanical valve. Now the first trial to explore this indication for one of the newer oral anticoagulants has been stopped.
In October, Boehringer told members of its speakers’ bureau that the post-surgery arm of the trial had been terminated because of “lower than projected plasma levels of dabigatran in this population, and an imbalance in reports of thromboembolic events (primarily strokes).” At that time, the company said the second arm of the trial would continue.
Dabigatran has been approved in Europe, but not in the United States, for venous thromboemoblism (VTE) prevention after knee- and hip-replacement surgery. Rivaroxaban (Xarelto) has been approved for both VTE prevention in the United States and Europe. To date there have been no head-to-head comparisons of the newer anticoagulants.
According to a recent study in Circulation: Cardiovascular Quality and Outcomes, dabigatran now has about 19% of the oral anticoagulant market, mostly for the approved treatment of AF “but increasingly for off-label indications” as well. A recent letter in the Journal of the American College of Cardiology provided information about the off-label use of dabigatran in two mechanical valve patients. Both patients developed thrombosis after switching to dabigatran from warfarin. The authors noted that “while there is a wealth of data and clinical experience on dosing and therapeutic response to warfarin in this context, these data are unavailable for dabigatran.” Although newer anticoagulants “hold tremendous promise for mechanical valve anticoagulation… there is a need for dose-finding studies and clinical trials to demonstrate safety and efficacy in this setting.”
December 12th, 2012
AMPLIFYING Treatment for DVT/PE
John Ryan, MD
The current guidelines from the American College of Chest Physicians (Kearon et al Chest 2012) and the Scientific Statement from AHA (Jaff et al Circulation 2011) favor 3-6 months of anticoagulation with no further treatment thereafter in unprovoked deep vein thrombosis/pulmonary embolism (DVT/PE), largely due to the risk of bleeding in the setting of prolonged warfarin therapy. Two studies have been published this year in NEJM that challenge this practice.
In the AMPLIFY-EXT study, Agnelli et al report that the use of apixaban, the oral factor Xa inhibitor, in patients with a history of DVT or PE for 12 months after 6-12 months of therapy with oral anticoagulants decreased the risk of recurrent DVT or PE or death compared to placebo. Two doses of apixaban were studied, both of which reduced the risk of DVT/PE/death from 11.6% with placebo to ~4%, depending on the dose of apixaban used (4.2% with 5 mg of apixaban or 3.8% with 2.5 mg of apixaban).
These results are similar to those observed in the WARFASA study published earlier this year and featured in an “Expert Is In” post here at CardioExchange. In that study, ASA 100mg was administered for 2 years after the discontinuation of anticoagulant therapy (and compared to placebo). DVT/PE recurred in 11.2% in those who received placebo over the 2-year period vs. 6.6% of the patients treated with ASA.
Taken together these articles reinforce the need for prolonged treatment in patients with unprovoked DVT/PE. More than 90% of the patients in the AMPLIFY-EXT study had unprovoked DVT/PE, and in the placebo group nearly 9% had a recurrent venous thromboembolism (VTE) or VTE-related death in the 12 months follow-up. With a 10% recurrence rate over the course of a year, DVT/PE should be managed as a lifelong condition, just the same way we manage ischemic heart disease.
- Are you planning to use apixaban or aspirin as extended treatment for your patients with DVT/PE??
- How long are you treating your patients with unprovoked DVT/PE?
December 11th, 2012
No Surprise: Smoking and Sudden Cardiac Death Closely Tied
Larry Husten, PHD
Although cigarette smoking has long been linked to cardiovascular (CV) disease and sudden cardiac death (SCD), the precise contribution of smoking, and the effect of smoking discontinuation, on SCD has not been clear. Now a new study from the Nurses’ Health Study published in Circulation: Arrhythmia & Electrophysiology provides new clarity about the relationship between smoking and SCD.
“Cigarette smoking is a known risk factor for sudden cardiac death, but until now, we didn’t know how the quantity and duration of smoking affected the risk among apparently healthy women, nor did we have long-term follow-up,” said lead investigator Roopinder Sandhu, in an AHA press release.
Dr. Sandhu and colleagues analyzed data from more than 100,000 women without known CV disease or cancer. During 30 years of followup there were 351 incident SCDs. Compared to women who never smoked, the risk of SCD was significantly elevated in current smokers (relative risk 2.44) and former smokers (RR 1.40).
The number of cigarettes smoked each day was correlated with the increase in SCD risk, but even women who smoked only 1-14 cigarettes per day had a significant 1.84-fold increase in risk. Women who smoked more than 25 cigarettes a day had a 3.3-fold increase. Smoking duration was also significant, resulting in an 8% increase in SCD risk for every 5 years of smoking.
Women who quit smoking reduced their SCD risk. After 15 years the reduction in risk achieved statistical significance, and by 20 years the risk was similar to women who had never smoked.
In an exploratory analysis, women smokers with coronary heart disease (CHD) had a much higher incidence of SCD than women without CHD. Women with CHD who quit smoking did not enjoy the same immediate reduction in SCD risk as observed in women without CHD.
“Sudden cardiac death is often the first sign of heart disease among women, so lifestyle changes that reduce that risk are particularly important,” said Dr. Sandhu. “Our study shows that cigarette smoking is an important modifiable risk factor for sudden cardiac death among all women. Quitting smoking before heart disease develops is critical.”
