July 19th, 2012
New ICD Lead Technology Creates New Set of Problems: A Perspective From One Electrophysiologist
Edward J. Schloss, MD
CardioExchange welcomes this guest post from Edward J. Schloss, MD, the medical director of cardiac electrophysiology at Christ Hospital in Cincinnati, OH.
Why I Don’t Like DF-4: A Personal Perspective
Since the first human implantable cardioverter defibrillator (ICD) implant in 1980, there have been a wealth of technological advances that have allowed these devices to become a standard therapy in the treatment of patients at risk for sudden cardiac arrest. Some of these, such as nonthoracotomy lead systems and biphasic waveforms, have revolutionized the industry. Others, such as downsized lead caliber, have added risk without clear benefits.
The DF-4 ICD lead is one of the latest technologies to be approved in cardiac rhythm management. DF-4 refers to the type of pulse generator connector on these leads. All three major U.S. ICD vendors have FDA-approved DF-4 ICD leads, and these have been generally well received by the implanting physician community. In this perspective, I endeavor to show that it may be appropriate to temper this enthusiasm.
Historical Background and Lead Design
Early ICDs required open-chest surgery to implant and were offered only to high-risk cardiac arrest survivors. In the early 1990s, transvenous lead ICD systems eliminated the need for thoracotomy or sternotomy. Soon thereafter, these leads were coupled with downsized ICD generators and advanced shock waveforms resulting in significant improvements in implant safety and success. As device indications expanded into primary prevention after the MADIT and SCD-HeFT trials, ICD implantation exploded in the U.S.
Over the years, a variety of industry standards have evolved to connect ICD leads to pulse generators under the guidance of the International Organization for Standardization (ISO). For most of the modern ICD era, we have been using ICD leads that have the DF-1/IS-1 connector. A representative DF-1/IS-1 lead is shown in figure 1:
In the figure, note the pulse generator header connections are on the top right and the connection to the ventricular endocardium is in the bottom left. The header connectors consist of one low-voltage pacing/sensing connector, termed IS-1, in the middle, and one or two high-voltage shock connectors, termed DF-1, alongside. These come together into a yoke that integrates the conductors into a single lead body as it enters the vasculature.
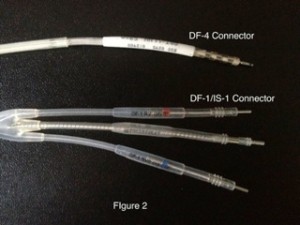
DF-4 was designed as an independent standard, combining all conductors into a single connection to the ICD header. In 2009, the first DF-4 ICD lead was FDA approved and marketed by St. Jude Medical. In the U.S., Medtronic and Boston Scientific also have available DF-4 ICD leads. Pictured in figure 2 is a DF-4 connector above a DF-1/IS-1 connector:
Note that the DF-4 lead has only one connection and no yoke. In addition, there are no plastic sealing rings on the lead connectors, as these are now integrated into the pulse generator.
At implant, these lead connectors are attached to an ICD pulse generator. The lead body is coiled behind and closed within the device pocket as shown in figure 3.
Doctors have embraced DF-4 technology and, according to some industry personnel, adoption has exceeded expectations. Potential advantages to DF-4 include:
- Easier implant with only one connector to attach rather than two or three
- Less likelihood of inadvertent misconnection to wrong port
- Easier dissection of lead at device replacement with less potential for lead damage and shorter procedure times
- Less pocket bulk
- Fresh sealing rings with each new pulse generator
At the 2012 Heart Rhythm Society Meeting in Boston, Dr. Michael Mirro reported the first long-term experience with DF-4 ICD leads. In the St. Jude SJ4 Post Approval Study, a 1700 patient experience over 5 years showed excellent performance with high satisfaction among implanting physicians.
So What’s the Problem with DF-4?
DF-4 ICD leads have a variety of known and potential disadvantages that must be weighed in the decision whether or not to use in a given patient. Some of these relate to the loss of flexibility in connectivity, others are due to the inherent uncertainty that comes along with a new lead design.
At ICD implant a DF-4 lead limits the ability to address high defibrillation thresholds (DFT). With DF-1/IS-1 leads, it is straightforward to add a supplemental shocking coil or array to the ICD lead system and plug this directly into the header block. With a DF-4 system, a high DFT would require replacement of both the ICD lead and pulse generator to allow addition of a new shocking lead. High DFTs are an infrequent but very important problem, and DF-4 takes away our flexibility in handling these patients. This year, Cogert et al published a case report of this scenario in PACE with the warning “until additional connectivity is added to the DF-4 defibrillator, we caution against selecting this technology in patients at risk for high DFTs.”
Isolated failure of the pacing/sensing component of an ICD lead is a common problem, especially with established leads not on recall. This can occur due to fracture of that element of the ICD lead or because of problems at the lead/myocardial interface (exit block). With DF-1/IS-1 leads, adding a dedicated pacing lead and substituting this in the header is a straightforward fix. Unfortunately, it is impossible to revise a DF-4 system in this fashion. Rather, an entirely new ICD lead would need to be implanted and the old lead abandoned or extracted.
Downgrade of an ICD to a pacemaker is straightforward with DF-1/IS-1 systems. This procedure is sometimes appropriate in patients no longer needing or desiring defibrillation backup at battery depletion but still having pacing indications. We offer this commonly to our elderly or ill CRT responders, and anyone else in whom ICD therapy is no longer appropriate or desired, who still need pacing therapy. Unfortunately, those with a DF-4 system will not have an easy path to downgrade due to the lack of a DF-4-compatible pacemaker system.
All of the above concerns could be addressed with new adapters or novel devices with modified header blocks. Unfortunately, there does not seem to be much movement from industry to address these issues. An adapter has been developed that will address the high DFT issue, but this has not yet been submitted for U.S. FDA approval. There have been no low-voltage adapters or modified headers developed yet, and none on the horizon. Industry personnel tell me that the high development cost and long approval cycle have been significant barriers. New restrictions from the FDA will likely have the unintended effect of slowing or halting the development cycle of these hardware fixes.
Over time we may see other unanticipated problems with DF-4 ICD leads. In an excellent review on this topic in April 2012 in Europace, Sticherling and Burri express concern that the complex design of the header/lead interface could lead to sensing or electrical isolation problems. Anyone quick to dismiss this potential problem need only think back on our humbling recent experiences with ICD failures to recognize that the late manifestation of an unanticipated failure mechanism is a fact of reality in cardiac rhythm devices.
To summarize, DF-4 disadvantages include:
- Inability to add additional shock electrode in setting of high DFT
- Inability to add pacing lead to remedy ICD lead fracture or pacing exit block
- Inability to downgrade ICD to pacemaker in future
- Uncertain long-term performance of DF-4 connector
Most of the perceived advantages of the DF-4 standard appear to be for the benefit of the doctor, not the patient. Even those advantages are pretty minimal. At initial implant, cutting back the number of connectors to attach may shave perhaps 30 seconds off the implant time. At device change, DF-4 may take a few minutes less to dissect out.
DF-4 does appear to reduce the incidence of improperly deployed setscrews and lead/header mismatch (abstract). One should recognize, however, that this rare problem should be 100% avoidable by a careful implanter and support staff with any lead system. It really shouldn’t be too much for our patients to expect us to put the right lead into the right connector and properly tighten three screws. A simple lab checklist would be a better and cheaper solution than designing and implementing a whole new lead technology.
Reduction in pocket bulk with DF-4 has been touted, but this is also a modest benefit at best. Observe in figure 3 above that an ICD lead properly wound into the pocket will hide its bulk behind the pulse generator and may not be noticeable. In addition, despite the loss of two connector ports, ICD pulse generator volumes are no smaller across vendors (EuroPace article, table 2).
Applying care and reason to the decision to use DF-4, there may be only a limited patient population in which benefit seems to outweigh risk. For my purposes, I would most likely consider DF-4 in a patient with the combination of being thin (to minimize pocket bulk), elderly or inactive (less likely to have lead fracture), without pacing indications (unlikely to need device downgrade in future), and without marked LV dilatation or very low EF (less likely to have high DFT or pacing threshold problems).
It is critical to adopt any new technology in a measured and thoughtful fashion. Experience has taught us time and again that newer isn’t always better. Determining true risks and benefits can be challenging, as we tend to learn all risks only years after product release. Because implantable devices, especially leads, are not easily replaceable, we need to anticipate potential problems while we wait for supportive long-term data. I’ve often said that the most valuable attribute in a pacemaker or ICD lead is a long, reliable track record.
Industry needs to prioritize patient safety in product design and marketing. DF-4 leads have been released and popularized even in the face of known deficiencies. Equal priority has not been applied to the development of adapters or modified pulse generators that we need to address predictable problems. Until we have these hardware fixes, I fear DF-4 will remain an example of a product designed more for the benefit of the doctor than the patient.