May 8th, 2014
Orbital Atherectomy Revisited
Kush Agrawal, MD
My attendance of the Cardiovascular Research Technologies (CRT) symposium — held in Washington, D.C. in February — was an exciting experience and a conference that I would highly recommend. Broad topics were channeled into streams of focus: complex PCI, lesion prep, peripheral intervention, and emerging applications towards endovascular approaches for critical limb ischemia.
While attending, I was able to observe and participate in a live case demonstration of orbital atherectomy, currently available in the form of the Diamondback Orbital Atherectomy System (OAS) manufactured by Cardiovascular Systems, Inc.
Where does OAS stand? Post-approval experience is limited, as the device was approved by the FDA last October, with individual operators endorsing an ease of use with the device, an enhanced fidelity of 1:1 burr to console feedback vs. traditional rotational atherectomy (RA), shorter fluoroscopy times, and lower contrast volume use.
For the observer, CRT’s unique approach to the live case demonstration of complex cases afforded an enhanced educational experience that should be modeled in future conferences.
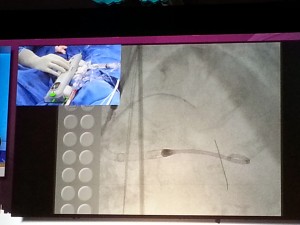
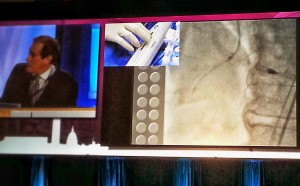
Dr. Jeffrey Moses from Columbia University led an expert panel (located on the right in the photograph below).
The live demonstration — on two large high definition (HD) projection screens — was performed by Drs. Samin Sharma and Annapurna Kini and colleagues at the Mt. Sinai cath lab (on the right screen in the photograph below), with preparatory educational slides (on the screen to the left), consistent with the pre-existing live case format available to the community on the Mt. Sinai website (e.g., coronary cases and peripheral cases).
The third screen on the far left (not pictured) contained a real-time Twitter-format feed for audience Q&A and participation. This was accomplished by co-opting the use of individual smartphones and tablets, seamlessly integrated through the CRT conference website.
This setup enabled the demonstration to maintain a focus that was continually redirected by the audience questions and afforded us a chance to provide answers to questions outside of the expert panel in real time.
The senior Mt. Sinai operators presented a complex case of a septuagenarian with recurrent angina who refused CABG. The patient had an LVEF of 30% and 3-vessel obstructive CAD, a highly calcified 80% proximal to mid LAD heterogeneous lesion, a patent mid left circumflex stent, and an ostial dominant 40% RCA with 50% at the mid-vessel. With the patient under monitored anesthetic care, the case initiated with bilateral femoral access with a 7F sheath on the right and a 9F sheath on the left and an Impella CP® (Abiomed) was inserted.
Lesion prep of both the LAD and RCA (no prior FFR or viability data were available at time of presentation) with the OAS appeared technically simple and straightforward in the hands of the skilled operators.
OAS currently costs about twice as much as traditional RA. Do you see it eventually supplanting traditional RA or as simply a “new toy” with a niche application?



I performed the first case in the Midwest after FDA approval. I was impressed by the ease of use and overall performance. Did a very nasty right that my partner attempted three weeks prior, couldn’t deliver a stent and are up a type b dissection. Overall a shallow learning curve if you used it in the periphery. The key is actually changing the way we used to perform atherectomy. Forget your picking back and forth motion; the device requires a slow 1-3 mm/s gentle forward maneuver. The nice thing is that unlike rota, you are shaving calcium on the way back. With the upcoming generation of the device, the crown will look like the 1.25 mm micro which is available in the periphery in addition to have diamond crystals on the leaving edge, which will simplify the management of ostial and bifurcation a lesions; currently you have to wedge the leading edge into the lesion before spinning whether in the ostium or at the bifurcation. Overall, the cost of the device will stop with the wider release. Will it supplant Rota? Possibly but I would hold off till the next generation of the device has been released. 15 caees at my site and no reflow or significant complications so far!