November 16th, 2010
What Does BASKET PROVE Have to Prove?
L. David Hillis, MD and Richard A. Lange, MD, MBA
What to make of new findings that DES are just as good as BMS for treating lesions in large coronary arteries? David Hillis and Rick Lange provide a brief tour of the relevant issues.
Getting a handle on the study…
Previous data suggested that the use of DES in large native coronary arteries confers no benefit and may, in fact, cause late harm due to stent thrombosis.
In BASKET PROVE (BAsel Stent Kosten Effektivitäts Trial – PROspective Validation Examination), 2314 patients needing stents that were 3 to 4 mm in diameter were randomly assigned to receive (a) a first-generation sirolimus-eluting stent (Cypher Select, Cordis), (b) a second-generation everolimus-eluting stent (Xience V, Abbot Vascular), or (c) a bare-metal (Visions, Abbott Vascular) stent. All patients were treated with aspirin and clopidogrel for 1 year.
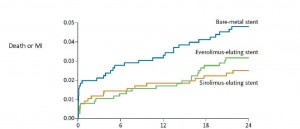
At 2 years, the rate of nonfatal MI or cardiac death did not differ significantly among the three groups. However, the rate of target-vessel revascularization was significantly lower in patients who received either DES than in those who received a BMS.
| Cardiac Death or MI | TVR | |
| BMS | 4.8% | 8.9%* |
| Sirolimus DES | 2.6% | 3.7% |
| Everolimus DES | 3.2% | 3.1% |
* p < 0.007 comparing BMS to either DES)
During the first 6 months after stenting, a statistically nonsignificant trend toward a lower rate of cardiac death and MI was seen with both DES, compared with BMS. However, since the clinical event rate was lower than expected, the trial was underpowered to detect small differences.
Is my patient a” basket case”?
One nice thing about BASKET PROVE findings is that they apply to a “real-world,” rather than a highly selected, patient population. One third of the patients had stable angina, one third had acute coronary syndromes, and one third had acute STEMI. No limit to the number of coronary arteries treated or stents used was imposed, and 76% of stents were implanted in subjects with “off-label” indications (e.g., more than one lesion per vessel, lesions in two or more vessels, lesions that were >27 mm in length, bifurcation lesions, chronic total occlusions, or acute coronary syndromes).
How do I weave these findings into my practice?
You can feel comfortable in the knowledge that there’s no harm in using DES rather than BMS in large coronary arteries (keeping in mind that the results of this study only apply to vessels 3 to 4 mm in diameter, not to smaller or larger arteries).
Should I be implanting DES, rather than BMS, in all large coronary arteries?
It’s not a slam dunk. For patients who cannot (or will not) take dual antiplatelet therapy for a year, BMS should be used. In the remainder, although it’s not harmful to use DES rather than BMS, it may not be cost effective. We need an analysis to examine whether the modest reduction in TVR (absolute reduction of 6 to 7%) justifies the increase in cost of DES over BMS.
Readers: What kind of stents do you generally recommend for large-artery stenoses, and will these findings cause you to reconsider your practice?

DES in large left main, LAD, ostial LCX or RCA, or in diabetics, otherwise BMS
Sirolimus stent 2 year mortality is 2.6%.
Bare metal stent 2 year mortality is 4.8%
So the difference is not statistically significant at the p=0.05 level. This DOES NOT MEAN there is no difference in mortality. It just means this study is underpowered to prove the significant difference. No way anyone would put a bare metal stent in me if I had a significant coronary stenosis and needed a 3 or 4 mm stent. The conclusion that this study indicates no difference is patently absurd on its face. Hopefully not too many patients will die because of this completely illogical conclusion.
If 100,000 patients with this type of disease get the bare metal stent instead of the sirolimus coated stent this study suggests that there is a high probability that 2,200 people will die unneccessarily over the course of two years.
Competing interests pertaining specifically to this post, comment, or both:
None.
I afraid,BASKET-PROVE’s virtually that “basket” of industry as well as over-enthusiastic interventional cardiologist’s basket will remain empty in future!But I am confident that, if this data as well as previously published same out-come data, if combined, our patient’s basket will not be empty!
It is consistent that the story of different stents(drug-eluting or bare-metal) stuck-up at repeated revascularization! Failed to provide meaningful benefit at ‘hard end points'(MI,Stroke,Cardiac death & All-cause mortality etc).Stents are for Angina relief nothing else!
At the end, high risk patient like diabetes,have two options.Either ‘stringent’ medical treatments(with ACEIs,B-blockers,Statins & Anti-platelet agents)plus vigorous lifestyle change(examples,regular 30-min brisk walking,maintain optimal body-weight,5 or more serving of fruits & green-leafy vegetables,whole grain cereals,fish or poultries,low-fat dairy products,limit salt intake, refined carbohydrate & trans-fatty acid etc.) Or CABG!!
I am very sorry for interventionist & tech-industry alike!
Happy to see a trial that did not include TVR in the primary outcome.
Dr. Schneider:
Actually, 2 yr total mortality for the stents was similar (sirolimus-eluting stent 3.6%, everolimus-eluting stent 3.2% and bare metal stent 4.4%: NS).
The clinical outcomes at 2 yrs in BASKET PROVE are …
stent (SES)
stent (EES)
(BMS)
(P value)
( P value)
While it’s possible that the study was underpowered to detect a clinical difference between the BMS and DES (as pointed out by Dr. Schneider), it is just as plausible that a larger study would have confirmed that there was no significant clinical difference between BMS and DES. Why is this? Previous studies comparing stents have not demonstrated a survival benefit of DES over BMS, even in “high risk patients” (i.e., those with small arteries, diabetes, and acute coronary syndromes). In addition, the graph showing the combined endpoint of cardiac death or MI (see below) shows that the “potential benefit” occurs in the first week of stent implantation. There’s no plausible reason for this, as previous studies have not shown a dramatically increased risk of early stent thrombosis with BMS, as compared to DES.
Dr. Lange,
Thanks for pointing out the endpoint was MI plus death. So, as you point out as well the difference is mostly in the first week after implant. “No plausible reason” is a cause for concern. I think this study raises more issues than it answers. The major endpoint is still almost twice as high for BMS as for Sirolimu DES, 4.8% vs 2.6%. This is a huge difference even if it does not achieve statistical significance at the p=0.05 level, and it cannot be ignored. There is a large conceptual difference between saying something is not different and saying that the difference does not achieve statistical significance in a given study. There were fewer than 800 patients in each arm of the study.
In comparison, the GUSTO trial in the 1980’s, declared that the approx. 6.8% mortality in AMI treated with IV TPA was statistically significantly less than the approx 7.5% mortality with IV streptokinase. This led to the almost complete substitution in the US of TPA for SK. The reason the smaller percentage difference in the GUSTO trial was statistically significant and the larger percentage difference in this trial is not statistically significant is that the GUSTO trial had 44,000 patients in two treatment arms and this trial had only 2314 patients in three treatment arms.
PS. I see you have a degree in MBA. I have a degree in physics and mathematics.
I couldn’t agree more that the difference — even if not statistically different — cannot be ignored. Interestingly, the study was performed because of previous ones suggesting that DES were associated with worse outcomes than BMS in large coronary arteries. The one thing on which we can all agree is that BASKET PROVE unequivocally demonstrated DES are not inferior to BMS in large coronaries. Whether or not they are better (i.e., lower death and MI) is not known. A larger study with longer follow-up would is needed to address this.
A degree in physics and mathematics trumps an MBA.
Having read this blog, my mind became “haunted” by probability and statistics. Should one ignore the results showing no statistical significance? Does it mean that that the result means no difference between the arms? Yet there is certain difference of some importance though it is NOT statistically important. If I should take this fact into account and the previous reasoning is correct, what is the statistics good for, then?
1/ The results in the BASKET-PROVE study did NOT reach statistical significance, therefore the differrences between DESs and BMS may have occurred by chance. (Statistics: A P<0.05 means that there is a less than 5% chance that the difference could have occurred by chance.) Both DES showed superior efficacy compared with BMS /it reads in pesentation slide-set/. Yet this superiority was not statistically significant. What is the real weight of evidence then?
2/ One of the commentators tries to calculate the number of TVRs with using bigger number of patients than the number studied. One cannot do this with having in mind that the result obtained int the BASKET-PROVE study is the statistical number and statistics is about probability. Therefore the result of simple multiplication is not exactly the number that would have beed reached with the bigger number of patients studied. Due to the lack of statistical significance, the result might differ either to small or great extent or reach statistical significance in the end.
WHAT IS THE SIGNIFICANCE OF “NOT SIGNIFICANT RESULT” THEN?
Is there anybody willing to explain the issue (see my pervious blog).
GIVE ME A LESSON, PLEASE.
Thank you very much.