June 25th, 2012
What Constitutes a Professional Society’s Endorsement?
Harry Peled, md
One of the doctors I work with in my role as Medical Director of Inpatient Cardiology at St. Jude Medical Center in California requested that a patient of his be given an externally wearable cardioverter-defibrillator as primary prevention after hospital discharge. I had previously seen such a device used only in very high-risk patients or for secondary prevention — i.e., patients who do not meet ICD guidelines.
Currently, the only FDA-approved wearable cardioverter-defibrillator on the market is LifeVest, made by Zoll. So I contacted a Zoll representative to ask for studies about it in primary prevention. The rep sent me the Medicare coverage guidelines but no RCTs or society guidelines. After more inquiries, I eventually received from one of the senior reps a communication that included this paragraph:
FROM THE ZOLL REP
Bruce Wilkoff, MD, FHRS, CCDS President of the Heart Rhythm Society, and David R. Holmes, Jr., MD, FACC President of the American College of Cardiology, have written a public letter addressing the Medicare guidelines supporting the LifeVest wearable cardioverter defibrillator (WCD) as the only solution. The LifeVest provides a customized, non-invasive protection with a 99.2% survival rate (Chung study) and an 80% mortality reduction in post PCI patients as cited by Zishiri.
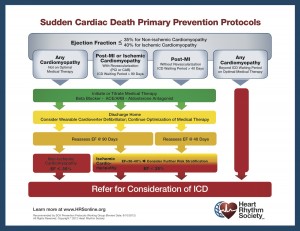
Given the reference to the Heart Rhythm Society (HRS) president, I dutifully went to the HRS website and found the HRS flow-chart protocol for sudden cardiac arrest (shown on the right). The flyer explicitly says to “consider” the wearable cardioverter-defibrillator and bears the HRS logo, along with language saying “recommended by SCA Prevention Protocols Working Group.”
Then I read a related disclaimer about the flow chart on the HRS website. Given the HRS logo on the protocol and the odd language of the disclaimer (which I discuss below), I decided to write to the HRS and describe my confusion and concerns.
MY EMAIL TO THE HRS
I am writing to inquire about the attached protocol for the prevention of sudden cardiac death, bearing the logo of the Heart Rhythm Society in the bottom right corner. The protocol indicates that a wearable cardioverter-defibrillator should be considered for patients who are discharged home and meet the referenced criteria.
I have read the related disclaimer on your website, which mentions the HRS SCA prevention working group that signed off on this protocol. However, there is no information about how this working group was chosen or why its recommendations are being disseminated. Given that a wearable cardioverter-defibrillator is not a guideline-recommended therapy (even though it is FDA-approved), this seems to me a curious element to include in a protocol.
The only wearable defibrillator on the market is the LifeVest, made by the Zoll Medical Corporation. It seems to me that the referencing of this therapy in your published protocol (bearing the HRS logo) is likely to lead many physicians to assume that the HRS endorses this therapy, website disclaimer notwithstanding. In effect, it is an endorsement of the LifeVest product without an adequate evidence base to support the recommendation. The inclusion of the logo on the protocol with an online disclaimer is, in my view, potentially confusing to clinicians like me and those I oversee. Clinicaltrials.gov does refer to 2 ongoing RCTs in this population.
Within a few days, the HRS’s Vice President of Marketing, Communications & Membership replied. I appreciated that the HRS took the time to offer a response, which is reproduced below with their express permission.
THE HRS RESPONSE
Thank you for your inquiry regarding the Sudden Cardiac Arrest (SCA) Primary Prevention Protocol. The document was produced as an educational tool to provide one possible care pathway for general cardiologists, primary care physicians, internists and other non-EP clinicians evaluating patients at risk for sudden cardiac death.
The suggested pathway states that, since the wearable cardioverter defibrillator (WCD) is an FDA released medical device with defined indications, it is a clinical option for consideration by physicians assessing risk for SCA. This pathway should NOT be viewed as a guideline-based recommendation on the part of HRS in support of the use of WCDs. Ultimately, use (or lack of use) of a WCD should be at the discretion of an individual practitioner, based on careful assessment of each patient’s individual risk for SCA by the treating physician.
And that’s all the HRS said. In effect, the HRS never explained to me who the SCA working group was or why an HRS logo appears on educational material that is “recommended” by the working group. I believe that any reasonable person would assume that the flow chart is officially HRS-endorsed, disclaimer or not — especially since the disclaimer is not on the educational flyer itself. So I remain puzzled and wonder if you are, too.
What are your thoughts about the flow-chart SCA protocol, the appropriateness of having the HRS logo on it, and the HRS’s response to Dr. Peled’s inquiry?

Reminds me of the AMA and Sunbeam in the 90’s. Wonder how much financial support HRS receives from Zoll.
Reminds me of the ADA and toothpaste. Only this time there is only one brand to endorse. Sounds like they are “dancing” around this one. Seems kind of strange letting someone place official logos for a specific product on a set of guidelines. If there was payment received, or some other type of support in exchange for use of the logo, generally I would interpret that as endorsement.
Makes me wonder what evidence the FDA used to approve this device-doesn’t sound like they are doing their job in protecting the American citizens from unproven claims of efficacy.