April 26th, 2012
FDA Advisory Panel Gives Green Light to HeartWare Ventricular Assist System
Larry Husten, PHD
 The FDA’s Circulatory System Devices panel voted 9-2 on Wednesday to recommend approval of the HeartWare Ventricular Assist System as a bridge to heart transplantation for patients with end-stage heart failure. The panel agreed unanimously (11-0) that the new device is effective. The panel was more divided about safety but ultimately voted 8-3 that the device was safe.
The FDA’s Circulatory System Devices panel voted 9-2 on Wednesday to recommend approval of the HeartWare Ventricular Assist System as a bridge to heart transplantation for patients with end-stage heart failure. The panel agreed unanimously (11-0) that the new device is effective. The panel was more divided about safety but ultimately voted 8-3 that the device was safe.
In briefing documents published online earlier in the week, FDA reviewers raised a number of questions about the safety of the device and the reliability of the data from the pivotal ADVANCE trial, in which 140 patients received the Heartware device and were compared to historical controls who had received Thoratec’s HeartMate II device. The FDA had not previously permitted historical controls to be used in this fashion. Safety questions focused on stroke and thrombosis.
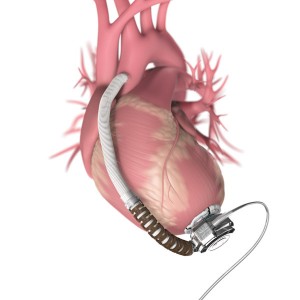
Unlike the HeartMate II VAS, which is surgically implanted in an abdominal pouch, the HeartWare VAS is implanted next to the heart.
“I think this device is of incredible benefit to patients who are very ill,” said panelist Ralph Brindis, as reported by MedPage Today. Acting panel chair Rick Page said the device represented “a real advance in technology,” as reported by Heartwire.
