May 9th, 2011
Higher Periprocedural Risk for Stroke Found in Women Undergoing Carotid Stenting
Larry Husten, PHD
The periprocedural risk for stroke is higher among women undergoing carotid artery stenting than among those undergoing carotid endarterectomy, according to new results from the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) published online in the Lancet Neurology. The same pattern was not found in men.
Last year, the main results from CREST showed no overall difference in the primary endpoint of death, stroke, or MI within 4 years after either stenting or endarterectomy, although a higher rate of stroke was found with stenting while a higher MI rate was found with endarterectomy.
In the new analysis, there was no significant difference in the primary endpoint in either men or women:
- men: 6·2% for stenting versus 6·8% for endarterectomy, HR 0·99, CI 0·66-1·46
- women: 8·9% versus 6·7%, HR 1·35, CI 0·82-2·23
However, in the periprocedural period (within 30 days after the procedure), women – but not men – who were assigned to stenting had a higher risk for events:
- men: 4·3% versus 4·9%, HR 0·90, CI 0·57-1·41
- women: 6·8% versus 3·8%, HR 1·84, CI 1·01-3·37
This difference appeared to be largely caused by an imbalance in the risk for periprocedural stroke:
- men: HR 1.39, CI 0.78-2.48, p=0.26
- women: HR 2.63, CI 1.23-5.65, p=0.013
In an accompanying comment, Martin Brown and Rosalind Raine write that “any differential effects of treatment by sex are unlikely to be explained by differences in sex chromosomes. Far more likely is that other patient characteristics are the true determinants of risk difference.”
May 9th, 2011
Words, ICDs, and Patient-Centered Medicine…
John Mandrola, MD, FACC
John Mandrola is a cardiac electrophysiologist and blogger on matters medical and general. Here is a recent post from his blog, Dr John M.
Guess what made the heart rhythm newswire yesterday?
It wasn’t a new medicine,
or a new stent,
not even a new ablation catheter,
and it surely wasn’t a revolution in motivating people to exercise.
It was words. Rhetoric… It seems that one man, Dr. John Wilson, read all of the major implantable cardioverter defibrillator (ICD) trials, dating back more than a decade, and found that the study authors emphasized positive aspects of ICDs while giving less weight to their sobering complication rates.
As reported by Steve Stiles on The Heart.org, Dr Wilson likened such “message framing” to marketing strategies that try to sell a product.
Though his opinions bordered on the sensational, Dr. Wilson’s advice spoke strongly to me, as here:
“I think the bulk of the information from these trials would suggest that these devices do make people live longer, but I think it’s also very likely that if patients were given a more balanced view about risks and benefits, fewer of them would be willing to take it on.”
And here (emphasis mine),
“Guidelines,” he said, “tend to make it sound like if something is found to be effective, it should be put in all patients with that problem, when in fact what should probably be said is: if it’s effective, patients should be given information about the benefit and the risk and allowed to use their own judgment to decide whether they’d prefer to live a little longer—in many cases just a couple of months longer—or possibly experience infection or get shocked multiple times with the defibrillator.” (If patients received this type of information), “you’d probably find that a lot of them would be very skeptical about having these devices put in.”
That last paragraph slants a little too far to the negative, but it suffices to show how a patient-centered ICD conversation is a tough one. It also highlights the notion of how practicing medicine gets harder as we accumulate better, and more invasive, tools (and provides yet another excuse reason why so many of us fall so woefully behind in the office).
In selected patients, ICDs unequivocally provide benefit. However, as with any invasive treatment, there are risks and alternatives, making ICDs akin to many other expensive and invasive therapies. Cancer chemotherapies (for example, adriamycin with its cardiac toxicity) come to mind.
Most smart doctors read journal articles with a critical eye. They (should) know that the writers are passionate and convinced of their positive findings. Such is human nature.
JMM
May 6th, 2011
News Reports Scrutinize Heart Rhythm Society’s Ties To Industry
Larry Husten, PHD
A series of investigative reports published on ProPublica analyzes the financial relationships between medical societies and drug and device companies. The Heart Rhythm Society, whose annual meeting is now taking place in San Francisco, comes in for especially close scrutiny. The series begins:
“From the time they arrived to the moment they laid their heads on hotel pillows, the thousands of cardiologists attending this week’s Heart Rhythm Society conference have been bombarded with pitches for drugs and medical devices.”
Other medical societies, including the ACC and the American Society of Hypertension, are also discussed in the series. In one item, Douglas Packer and Bruce Wilkoff, the HRS president and president-elect, answer in some detail numerous questions asked by the authors of the series. Another item raises questions about whether industry has exerted undue influence on information sheets for arrhythmia patients published on the HRS website.
May 5th, 2011
5 Patients Infected With Hepatitis C While Undergoing MPI
Larry Husten, PHD
Five patients undergoing myocardial perfusion imaging (MPI) at a single outpatient clinic in North Carolina were infected with the hepatitis C virus (HCV) during the procedure, according to a paper published online in the American Journal of Cardoliology. Public health officials from North Carolina and the CDC report that their investigation began in May 2008 when a patient not otherwise at risk for HCV infection tested positive for HCV RNA after a blood donation.
The investigators found that on 2 separate dates a total of 5 patients were newly infected with HCV. The likely cause of the infection was unsafe injection practices, due to the nuclear medicine technologist who “routinely drew up flush from multidose vials of saline solution using the same needle and syringe that had been used to administer radiopharmaceutical doses.”
The authors write that their “investigation adds outpatient cardiology practices to the growing list of venues where viral hepatitis transmission has occurred because of unsafe injection practices.” They conclude:
“Transmission of HCV in health care settings is entirely avoidable and could largely be prevented by increasing awareness of basic injection safety principles. In particular, health care providers should ensure that needles and syringes are never reused and should be cognizant of the risks associated with shared medication vials.”
May 4th, 2011
Study Estimates That Atrial Fibrillation Adds $26 Billion to Yearly U.S. Healthcare Costs
Larry Husten, PHD
Atrial fibrillation may add $26 billion to the nation’s healthcare bill, according to a study published in Circulation: Cardiovascular Quality and Outcomes. Michael Kim and colleagues compared insurance claims for 1 year from 89,066 AF patients with claims from controls matched for gender, age, and other medical conditions and found that AF results in a net incremental cost per patient per year of $8,705.
Most of the additional costs came from more frequent hospitalizations in the AF group: AF patients were twice as likely as controls to be hospitalized (37.5% vs. 17.5%) and three times as likely to have multiple hospitalizations (11.1% vs. 3.3%). Some $6 billion was spent directly on costs related to AF; $9.9 billion for non-AF cardiovascular care; and $10.1 billion went for noncardiovascular health costs.
“We’re not going to impact healthcare costs or cardiovascular outcomes by just addressing atrial fibrillation itself,” said Michael Kim, the lead author of the study, in an AHA press release. “The large amount of cardiovascular disease among atrial fibrillation patients appears to worsen outcomes and increase costs. This is a sicker population.”
Sanofi-Aventis, the manufacturer of the AF drug dronedarone (Multaq), provided financial and editorial support for the development of the manuscript of the paper.
May 3rd, 2011
Study Challenges Efforts to Lower Salt in the General Population
Larry Husten, PHD
A new study challenges the conventional wisdom that lowering salt intake in the general population will result in fewer cardiovascular events. In a paper in JAMA, Katarzyna Stolarz-Skrzypek and colleagues report the results of the study, in which they followed 3,681 European people without cardiovascular disease after measuring their blood pressure and urinary sodium excretion.
After adjusting for other variables, the researchers found that systolic (but not diastolic) blood pressure was independently correlated with sodium excretion, and that systolic blood pressure changed in accord with changes in sodium excretion. However, higher sodium excretion was not associated with more cardiovascular complications. In fact, the investigators reported “a weak but consistent inverse association between cardiovascular mortality and the 24-hour urinary sodium excretion at baseline.”
The authors note that most salt studies have measured salt intake, and no previous study has examined the “longitudinal association between changes in blood pressure on a continuous scale in relation to changes in 24-hour urinary sodium excretion.”
The authors conclude that their findings “refute the estimates of computer models of lives saved and health care costs reduced with lower salt intake. They do also not support the current recommendations of a generalized and indiscriminate reduction of salt intake at the population level. However, they do not negate the blood pressure−lowering effects of a dietary salt reduction in hypertensive patients.”
May 3rd, 2011
CABG Takes the Brunt of Decline in Revascularization Procedures
Larry Husten, PHD
In recent years, the overall revascularization rate in the U.S. has declined only slightly, but CABG rates have taken the brunt of the change, while PCI rates have remained relatively stable, according to a new study by Andrew Epstein and colleagues published in JAMA.
The researchers found that from 2001-2002 to 2007-2008:
- The annual rate of revascularization decreased significantly, but by only 15% (p<0.001).
- The CABG rate decreased significantly, by nearly 40%, from 1742 surgeries per million adults per year to 1081 (p<0.001).
- PCI rates did not change significantly (from 3827 procedures per million adults per year to 3667 procedures, p=0.74).
- The number of hospitals that provide CABG increased, resulting in a 28% decrease in the median CABG caseload per hospital.
The results, write the authors, “suggest the possibility that several thousand patients who underwent PCI in 2008 would have undergone CABG surgery had patterns of care not changed markedly between 2001 and 2008. Our data imply a sizeable shift in cardiovascular clinical practice patterns away from surgical treatment toward percutaneous, catheter-based interventions.”
May 2nd, 2011
Standard Guidelines Compared with Individualized Guidelines
Larry Husten, PHD
Should patients be treated by standard guidelines, or should guidelines be individualized for patients? In a study published in the Annals of Internal Medicine, David Eddy and colleagues used data from the Atherosclerosis Risk in Communities (ARIC) Study to calculate and compare the expected benefit from hypertension treatment based on JNC 7 guidelines with the expected benefit from treatment based on individualized guidelines. (Individualized guidelines were derived from a calculator developed as part of the Archimedes computer model designed to simulate clinical trials.)
The authors (all of whom are affiliated with the Archimedes project) found that “individualized guidelines could prevent more than an additional 43% of MIs and strokes than JNC 7 guidelines for the same cost.” To achieve the same benefit as the JNC 7 guidelines, 52% of people indicated for treatment by JNC 7 would not be indicated for treatment by the individualized guidelines, while 36% of people indicated for treatment by the individualized guidelines would not be indicated for treatment by JNC 7.
The authors conclude: “Our results show that individualized guidelines can prevent the same number of MIs and strokes at a lower cost (and by exposing fewer people to the risks and adverse effects of treatments) or can prevent more MIs and strokes at the same cost as the JNC 7 guideline.”
In an accompanying editorial, Douglas Owens notes that “tailoring recommendations is easier said than done.” He writes that “tailored recommendations must meet 3 challenges” before being generally accepted. First, for a specific clinical problem, individualized guidelines must be shown to have the potential to produce better results. Second, clinical trials must demonstrate these potential benefits. Third, the individualized guidelines must be “easy enough to use that clinicians will adopt them.”
May 2nd, 2011
FDA Approves New Drug for Type 2 Diabetes
Larry Husten, PHD
The FDA announced today that it has approved linagliptin, a new drug for type 2 diabetes. The DPP-4 inhibitor was developed by Eli Lilly and Boehringer Ingelheim and will be sold under the brand name of Tradjenta.
Linagliptin was studied in 3,800 patients with type 2 diabetes, the FDA said, and was better than placebo in controlling glucose. The drug has been studied as monotherapy and in combination with other drugs indicated for type 2 diabetes, but its use with insulin has not been tested and it should not be used in people with type 1 diabetes.
According to the FDA, the most common side effects of the drug are upper respiratory infection, stuffy or runny nose, sore throat, muscle pain, and headache.
May 1st, 2011
Don’t Miss the New AHA Recommendations on Triglycerides
Harlan M. Krumholz, MD, SM
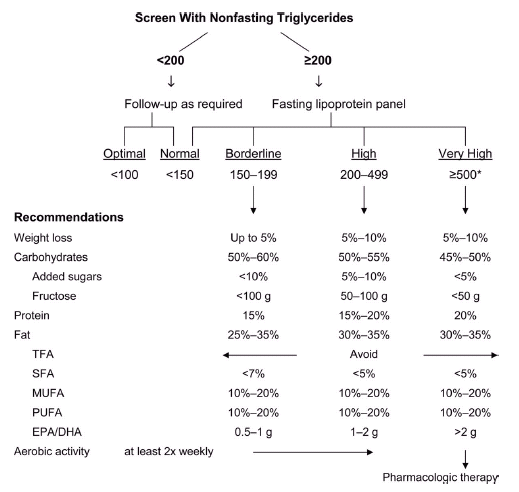
I’ve been surprised at the lack of fanfare surrounding the American Heart Association’s recently published scientific statement on triglycerides and cardiovascular disease (CVD). The attention it did receive focused on the lower fasting triglyceride level that is now considered optimal: <100 mg/dL. In my opinion, the real headline was the committee’s important statements in support of less drug treatment — in particular, the recommendation for a substantial increase in the triglyceride level that should trigger consideration of pharmacologic therapy.
After a careful review of the recent literature, the committee concluded that pharmacologic therapy should not be started until a patient’s fasting triglyceride level is ≥500 mg/dL (in contrast to the Adult Treatment Panel’s recommendation of ≥200 mg/dL). See the figure below, which also appears on page 17 of the AHA statement.
The AHA committee also explicitly acknowledges (on page 6) that “the independence of triglyceride level as a causal factor in promoting CVD remains debatable. Rather, triglyceride levels appear to provide unique information as a biomarker of risk, especially when combined with low HDL-C and elevated LDL-C.” This clear statement—together with the new, higher threshold for initiating drug treatment—represents a remarkable change.
Meanwhile, on April 20, Abbott announced that sales of its flagship fenofibrate drugs increased by 28% in the first quarter.
Two questions:
- Why are doctors prescribing fibrates with growing enthusiasm when data from negative drug trials support an increasingly conservative approach to drug treatment?
- Given the new AHA recommendations, what should we do about all the people who were started on drug therapy to lower triglyceride levels that were less than 500 mg/dL?
I welcome your insights.