May 26th, 2011
NHLBI Stops the AIM-HIGH Trial of Niacin
Larry Husten, PHD
The AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health) trial of niacin has been stopped early by the NHLBI. The trial was designed to test the addition of high-dose, extended-release niacin to statins in people at risk for CV events who had well-controlled LDL but low HDL and elevated triglyceride levels.
The trial was stopped early after a regularly scheduled meeting on April 25, 2011 of the data and safety monitoring board (DSMB) determined that there was no difference in the rate of clinical events between the two treatment groups. As expected, niacin was effective at raising HDL and lowering triglyceride levels, but it had no effect on the composite endpoint of fatal or nonfatal MI, strokes, hospitalizations for ACS, or the rate of revascularization procedures.
At an NIH press conference, the study director, Ruth McBride, reported that the annualized rate of the primary endpoint was 5.6% in the control group and 5.8% in the niacin group. In addition, there was a small and unexplained increase in the rate of ischemic strokes in the niacin arm. During the 32-month followup period, there were 28 strokes (1.6 %) in the niacin group versus 12 strokes (0.7%) in the control group. An NIH/NIHLBI press release noted that 9 of the 28 strokes in the niacin group “occurred in participants who had discontinued the drug at least two months and up to four years before their stroke” and that previous research has not raised a warning flag about an elevated risk of stroke associated with niacin use.
Starting in 2005, 3,414 patients were enrolled in AIM-HIGH, which was funded by the NHLBI with additional support from Abbott, which also supplied the niacin formulation (Niaspan) used in the study. The NHLBI noted in its press release that several previous trials, including the ACCORD trial with fenofibrate and the ILLUMINATE trial with torcetrapib, have failed to demonstrate the beneficial effects of HDL-raising drugs.
“This study sought to confirm earlier and smaller studies.,” said Susan B. Shurin, the acting director of the NHLBI, in the press release. “Although we did not see the expected clinical benefit, we have answered an important scientific question about treatment for cardiovascular disease.”
“The results from AIM-HIGH should not be extrapolated to apply to potentially higher-risk patients such as those with acute heart attack or acute coronary syndromes, or in patients whose LDL cholesterol is not as well-controlled as those in AIM-HIGH,” said William Boden, M.D, the co-principal investigator of the study.
At the NIH press conference, Boden said he would consider using niacin in an ACS patient with an LDL level of 140 and an HDL level of 25. He expressed concern that “there will be a temptation to interpret this trial as a quote unquote negative trial.”
In a statement, the FDA said it had “made no new conclusions or recommendations regarding the use of extended-release niacin alone or in combination with simvastatin or other statins” and said it would “conduct a comprehensive review of the AIM-HIGH trial data as soon as they become available to determine their impact on the approved indications for extended-release niacin.”
We appreciate all of your great comments about AIM-HIGH — both below and at our Voices blog, “AIM-HIGH Halted: Death Knell for for the HDL Hypothesis? Six Experts Weigh In.” To keep the discussion moving — but in one place — we’re closing out the thread below and hope that you will continue talking
May 25th, 2011
FDA Approves Xience Nano for Small Vessels
Larry Husten, PHD
The FDA has approved the Xience Nano everolimus-eluting stent, Abbott has announced. The stent is based on the same platform as the popular Xience V stent, and can be used in vessels as small as 2.25 mm. The approval was based on results from the SPIRIT Small Vessel clinical trial. The rate of target lesion failure was 8.1% in that trial, similar to the rate observed in the SPIRIT trials with Xience V.
May 24th, 2011
Copeptin May Help Predict CV Death in Elderly HF Patients
Larry Husten, PHD
The biomarker copeptin, which is a surrogate marker of vasopressin, may help predict the risk for death in elderly heart failure patients, according to a new study in JAMA. Urban Alehagen and colleagues followed 470 elderly HF patients from 1996 through 2009, during which time there were 226 deaths from any cause and 146 deaths from cardiovascular causes. They found that copeptin was an independent predictor of death and also contributed additional prognostic information when used in tandem with the established biomarker NT-proBNP.
Death from any cause occurred in 69.5% of patients in the highest quartile of copeptin versus 38.5% of patients in the lowest quartile (HR 2.04, CI 1.38-3.02). For NT-proBNP, the death rate was 75.9% in the highest quartile versus 28.3% in the lowest quartile.
CV mortality was 46.6% in patients in the highest quartile of copeptin versus 26.5% in patients in the lowest quartile (HR 1.94, CI 1.20-3.13). For NT-proBNP, the CV death rate was 56.9% in the highest quartile versus 15.9% in the lowest quartile.
Among subjects with low concentrations of both markers, the CV survival rate was 74.6%, compared with 23.0% in those with high concentrations of both markers.
The authors say their results “suggest that vasopressin may be a potential target for therapeutic intervention.”
May 24th, 2011
New-Onset AF Linked to Increase in Death and CV Events in Women
Larry Husten, PHD
In the Women’s Health Study, which followed nearly 35,000 women for more than 15 years, mortality was significantly higher in the 1011 women who developed AF than in the women who did not, according to a report by David Conen and colleagues published in JAMA.
Here are the incidence rates (per 1000 person-years of follow-up) for women who developed AF compared with women without AF:
- all-cause mortality: 10.8 versus 3.1, adjusted HR 2.14
- CV mortality: 4.3 versus 0.57, adjusted HR 4.18
- non-CV mortality: 6.5 versus 2.5, adjusted HR 1.66
The investigators observed that the increased mortality risk was “partly mediated through the occurrence of nonfatal cardiovascular disease,” in particular CHF and stroke. They concluded that this findings indicates “a potential opportunity to improve the outcome of individuals with new-onset AF through both prevention and optimal management of these associated comorbidities.”
In an accompanying editorial, Yoko Miyasaka and Teresa Tsang write that the study helps confirm that “AF is not a benign condition but in fact is associated with premature death. Newly identified AF in seemingly healthy women should be taken seriously and treated aggressively….”
May 24th, 2011
Stent BioWars: Erode or Absorb?
Richard A. Lange, MD, MBA and L. David Hillis, MD
In January 2011, we blogged about ABSORB, a bioresorbable stent, when it received CE approval for use in Europe.
Drug-eluting stents (DES) are composed of a metal scaffold that is coated with a polymer containing an antiproliferative agent , which is released gradually over the weeks to months after the stent is inserted. The durable polymer residue has been implicated as a cause of persistent arterial wall inflammation and delayed vascular healing, which may play a role in the occurrence of late stent thrombosis and restenosis (>12 months after PCI). If so, stent polymers that erode after drug release may potentially reduce late complications.
At the EuroPCR 2011 meeting, researchers presented findings of a meta-analysis of 3 studies (ISAR-TEST-3, ISAR-TEST-4, and LEADERS, each with 3 years of follow-up) of DES with either bioerodable polymers or durable polymers (i.e., Cypher). Compared with durable polymers, bioerodables were associated with less stent thrombosis (1.2% vs. 2.1%; P=0.013) and better outcomes (incidence of cardiac death, MI, and target lesion revascularization, 18.2% vs. 21.1%; P=0.04).
Late stent thrombosis and restenosis are uncommon occurrences; however, this meta-analysis provided sufficient patient numbers (2358 patients with bioerodable-polymer stents and 1704 patients with Cypher stents) to detect a statistically significant difference in outcomes.
If the incidence of late complications after DES implantation is reduced by making the polymer disappear, do you think that making the entire stent disappear will be a significant improvement?
May 23rd, 2011
What Is the Impact of Screening Low-Risk Patients with CT Angiography?
Larry Husten, PHD
In a study published online in Archives of Internal Medicine, John McEvoy and colleagues examine the impact of screening low-risk patients with coronary CT angiography (CCTA). They compared 1000 South Korean patients who underwent CCTA with 1000 matched controls.
CCTA identified 215 people with coronary atherosclerosis. At 90 days and at 18 months, statins and aspirin were being taken by a significantly higher percentage of patients in the CCTA-positive group than in the CCTA-negative group or in the matched control group:
At 90 days
- statins were used in 34% of CCTA-positive patients vs. 5% of CCTA-negative patients and 8% of those in the control group
- aspirin was used in 40% vs. 5% and 8%
At 18 months
- statins were used in 20% vs. 3% and 6%
- aspirin was used in 26% vs. 3% and 6%
The investigators also found that, at 90 days, there were more secondary tests and revascularizations in the CCTA group than in the control group:
- secondary tests: 5% vs. 2%, p<0.001
- revascularizations: 1% vs. 0.1%, p<0.001
At 18 months there was one cardiovascular event in each group.
The authors observed that in their study “we found that the evidence-free performance of CCTA in asymptomatic patients was associated with further evidence-free testing and interventions.” They concluded that their data “concurs with the prevailing notion that screening CCTA does not have a role in low-risk patients.”
In an invited commentary, Michael Lauer said the study “serves as a powerful reminder of the 2-edged effects of screening.” He continues:
“The only way to know whether screening by CCTA leads to clinically beneficial diagnosis of real disease, as opposed to pseudodisease, is by performing large-scale controlled trials, preferably with randomization. We cannot simply assume that just because a screening test predicts clinical outcomes, interventions necessarily will prevent them. Similarly, we cannot assume that because other tests diagnose disease that responds to treatment, a new screening test must do the same.”
May 23rd, 2011
Eplerenone Found to Also Reduce AF in Heart Failure Patients
Larry Husten, PHD
Results from EMPHASIS-HF (Eplerenone and Atrial Fibrillation in Patients with Systolic Heart Failure and Mild Symptoms) previously showed that adding the aldosterone antagonist eplerenone to standard therapy in patients with NYHA class II heart failure resulted in the reduction of the composite endpoint of death from cardiovascular causes or hospitalization for heart failure. Now, a sub-analysis of the trial presented by Karl Swedberg at the Heart Failure Congress 2011 in Gothenburg, Sweden suggests that eplerenone may have the additional beneficial effect of preventing atrial fibrillation (AF) in heart failure patients.
Among the 2737 patients randomized in the trial, 1794 did not have AF at the start of the study. In these patients, new-onset of AF occurred in 2.7% of eplerenone recipients versus 4.5% of placebo recipients (HR 0.58, CI 0.35-0.96, p=0.034).
The investigators also found that eplerenone had similar overall effects in patients both with and without AF at baseline and that AF at baseline did not significantly increase the risk for major study outcomes. However, the rate of total mortality or all-cause hospitalization was higher in the group of patients with AF at baseline than in the patients without AF at baseline (42.2% vs. 35.3%, p=0.008).
The discussant of the trial, Lars Ryden, said that in his opinion eplerenone should join ACE inhibitors and ARBs as recommended agents for the primary prevention of AF in heart failure patients.
May 20th, 2011
Data from Our International Survey of Medical School Grads
John Ryan, MD
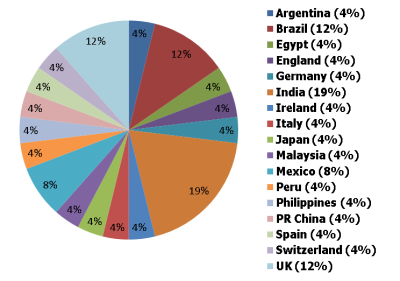
Earlier this year we at CardioExchange surveyed international medical school graduates from our online community. We sent out a questionnaire to 850 internationally based physicians from around the globe and received 29 responses (a 3.4% survey-response rate) from 6 continents. The largest group of respondents (19%) was from India, then 12% each from Brazil and the U.K., with the rest from countries as far ranging as Japan, Egypt, Peru, China, and Switzerland.
Where Our Survey Respondents Graduated from Medical School
Interestingly, although respondents received most of their cardiology training in their native countries, 59% went to the United States to complete it. However, nearly all of those who came to the U.S. said that they intend to return to their home countries eventually. That contradicts previous literature published by the University of Michigan (1995) showing that once international physicians come to the U.S. they tend to stay. However, both the age of those data and the size of our small, informal sample may be among the factors that account for the discrepancy between the two.
In our survey, a plurality of respondents (45%) said they had completed 2 to 4 years of postgraduate training before starting cardiology training. With regard to general cardiology training itself, most respondents (62%) indicated that their native countries require 2 to 4 years, but a substantial percentage (27.5%) said that 4 to 6 years of general cardiology training are required (considerably longer than in the U.S.). Perhaps a partial explanation is that in some U.K. models of Specialist Registrar training, general-medicine training years are built in to cardiology specialty training, thereby increasing the total time logged under cardiology.
About two thirds of our respondents indicated that their general cardiology programs provided training in diagnostic catheterization and echocardiogram interpretation, but a still-remarkable nearly one third said that their programs did not provide training in these modalities. Perhaps the desire to get such training explains why many trainees come to the U.S., but our data do not clarify that point.
Only about half of our respondents said that their native countries required board certification to practice as a cardiologist. As in the U.S., most of these board exams are taken 2 to 4 years after starting cardiology training, although they are a combination of written and clinical examinations (not computerized tests, as in the U.S.).
Work life after completing general cardiology training does not differ much between the U.S. and other countries, according to our respondents. They are, for example, permitted to perform admissions, do consultations, and read ECGs. Consistent with the finding that two thirds received training in diagnostic catheterization, 64% said they went on to do catheterizations in their practices. Interestingly, 84% of respondents describe echocardiogram interpretation as part of their practices, despite the fact that nearly 30% of all respondents said they had not received such training in their general cardiology programs. This disjuncture may be attributable, again, to having received training in the U.S. or, perhaps, to systems in their home countries that allow cardiologists to interpret echos without formal training.
Subspecialty training was available in all the countries represented by our respondents, and 72% planned to undertake it. The most popular subspecialty was interventional cardiology (38% of respondents), with most of the rest roughly evenly distributed among imaging, electrophysiology, research, and heart failure. Although three quarters of respondents interested in subspecialty training intend to do most of it in their home countries, close to half of all our respondents said that they intend to come to the U.S. at some point before their overall training is complete and then to return home.
A quarter of medical school graduates in the U.S. have education-related debt that exceeds $200,000, according to the Association of Medical Colleges (October 2007). In our international survey, about 80% of respondents had less than $6000 in debt accrued over the course of their training, and 43% reported having no educational debt at all.
Modest though our sample was, we’re wondering what your thoughts are about the data our international survey respondents shared with us. What insights do the numbers provide? Any surprises?
May 19th, 2011
FDA Panel Delivers Mixed Verdict on Trilipix (Fenofibrate)
Larry Husten, PHD
The FDA’s Endocrinologic and Metabolic Drugs Advisory Committee delivered a mixed verdict on fenofibrate (Trilipix, Abbott). On the one hand, the panel agreed unanimously that the FDA should require Abbott to perform a large clinical trial in high-risk patients with elevated triglyceride levels and low HDL levels who nevertheless have achieved target LDL cholesterol levels on statin therapy.
On the other hand, 9 panelists voted to keep (3 votes) or incorporate the findings from ACCORD (6 votes) in the current label. Four panelists voted to withdraw the indication.
May 18th, 2011
FDA Announces Details of Severe New Restrictions on Rosiglitazone
Larry Husten, PHD
The FDA has announced the details of the updated REMS (risk evaluation and mitigation strategy) for rosiglitazone, the embattled and highly controversial diabetes drug. The new REMS will sharply restrict access to and distribution of drugs containing rosiglitazone (Avandia, Avandamet, Avandaryl).
In order for physicians to prescribe and for patients to receive rosiglitazone, they will need to enroll in a special program mandated by the FDA, called the Avandia-Rosiglitazone Medicines Access Program. Rosiglitazone will now be restricted for use by patients already being successfully treated with the drug or by patients who have failed other anti-diabetic drugs and who do not wish to use pioglitazone.
After November 18, 2011, rosiglitazone will only be available by mail order through pharmacies participating in the program.
The FDA action follows an FDA advisory panel last summer and the subsequent FDA announcement in September that it would significantly restrict the use of rosiglitazone.
