June 8th, 2011
FDA Recommends Limiting Use of High-Dose Simvastatin
Larry Husten, PHD
The FDA today recommended significant limitations in the use of high-dose (80 mg) simvastatin because of the increased risk for myopathy. The FDA said the 80-mg dose should be used only in people who have been taking the high dose for at least one year and have had no evidence of myopathy. The high dose should not be started in new patients, including those already taking lower doses of the drug.
The FDA also said that the risk for myopathy is higher in the first year of treatment with simvastatin, is “often the result of interactions with certain medicines, and is frequently associated with a genetic predisposition toward simvastatin-related myopathy.” Hospitalization for rhabdomyolysis, the most serious form of myopathy, occurs in 4.9 people per 100,000 taking simvastatin for one full year, compared with an average rate of 4.4 per 100,000 taking statins in general.
The labels for drugs containing simvastatin, including Simcor and Vytorin, have been revised to include the new dosing restrictions, the FDA said. The agency advised healthcare professionals to switch patients who do not meet their cholesterol goal on 40-mg simvastatin to alternative treatments that provide a greater cholesterol-lowering effect.
June 7th, 2011
Scans or Scams? ProPublica Investigates Heart Check America
Larry Husten, PHD
ProPublica, the nonprofit public interest journalism site, has once again focused on a heart-related topic (for previous ProPublica reports see our stories here and here). This time journalist Marshall Allen reports the results of a detailed investigation of Heart Check America, a chain of imaging centers featuring electron-beam CT calcium scans.
The story begins with an anecdote as the journalist and his wife visit one of the clinics in a Las Vegas office park to hear a sales pitch from the company. The salesman cites numerous well-known cases of celebrities who had died suddenly:
“You never know when it could happen. … Boom, you’re dead!” he exclaimed, slapping a desk for emphasis.
If only they had come to Heart Check America, Tom said ruefully. The company’s Electron Beam Tomography machines could have spotted the harmful build-up of calcium in their arteries, indicating they were at risk. The company scanned other organs, too. Perhaps a test could have helped Patrick Swayze, who died of pancreatic cancer, Tom said.
After 45 minutes, Tom got down to business. He pulled out a price sheet and urged us to go beyond the free scans and upgrade to a 10-year contract for annual imaging services, including heart, lung, bone-density and other scans. If we signed up immediately, the contract – usually $7,995 – could be ours for just $2,995 plus $199 in annual dues. Financing was available on the spot.
The ProPublica story continues with a detailed investigation of the company, outlining the company’s multiple clashes with state regulators, consumer watchdogs, and medical experts. The company, which was founded in 1992, had been in a long decline until 2008, when a new leader with a questionable background in timeshares, David Haddad, reinvigorated the company, adding new centers and employing “marketing techniques similar to those [he] had used in his timeshare businesses, calling consumers at home and offering them free heart scans to come listen to sales presentations.”
The article quotes several cardiologists, including William Zoghbi, president-elect of the ACC, who told ProPublica that calcium scans “are best suited to people who exhibit some possible indications of heart disease, such as high cholesterol, or who have a family history of premature coronary disease.”
Another cardiologist, Matthew Budoff, was listed by Heart Check America as the medical director of one of its sites, but told ProPublica that he had no affiliation with that particular unit (although he is the medical director of another Heart Check unit). “It’s a little disconcerting to hear that I’m affiliated with a site that I’m not familiar with,” said Budoff.
But Budoff continues to endorse EBCT, telling ProPublica that “he’s ‘very concerned’ that the controversy surrounding Heart Check America could dissuade people from getting scans that could identify heart disease.”
“We have to separate out a single provider from the test itself,” he said. “The test is good. The provider may not have done something proper.”
But the chairwoman of the U.S. Preventive Services Task Force says that “the scientific evidence is just not there one way or another,” and says that the company is “preying on the fears of the public.”
June 6th, 2011
Smoking Found to Be ‘Potent’ Risk Factor for Symptomatic PAD in Women
Larry Husten, PHD
The latest report on the 40,000 women enrolled in the Women’s Health Study provides further demonstration that smoking is a “potent” risk factor for symptomatic peripheral artery disease. The paper, by David Conen and colleagues, appears in the Annals of Internal Medicine.
Here are the age-adjusted incidence rates per 1000 person-years of follow-up:
- never smoked: 0.12
- former smoker: 0.34
- <15 cigarettes per day: 0.95
- >15 cigarettes per day: 1.63
Here are the age-adjusted hazard ratios:
- never smoked: 1.0
- former smoker: 2.95 (1.91-4.55)
- <15 cigarettes per day: 8.76 (5.05-15.21)
- ≥ 15 cigarettes per day: 16.51 (10.66-25.57)
Adjusting for other risk factors did not substantially change the results. In their conclusion, the authors noted that “although smoking cessation dramatically reduces the risk for PAD, an increased disease risk remains even after long-term smoking cessation, which demonstrates the importance of both prevention of smoking initiation and efforts to promote long-term abstinence.”
June 5th, 2011
PARTNER A Results Published in NEJM
Larry Husten, PHD
Following its initial presentation at the ACC meeting in March, the results of PARTNER A have now been published online in the New England Journal of Medicine, in conjunction with a presentation at the Transcatheter Valve Therapies meeting in Vancouver. As reported previously, 699 high-risk older patients with severe aortic stenosis were randomized to either transcatheter aortic valve implantation (TAVI) or surgery for aortic valve replacement (AVR). Death at one year, the primary endpoint of the study, was 24.2% in the TAVI group and 26.8% in the AVR group (p=0.44), a reduction of 2.6% in favor of TAVI ,which met the predefined margin for noninferiority. The PARTNER investigators also reported:
- Major stroke at 30 days: 3.8% for TAVI vs. 2.1% for AVR (p=0.20)
- Major stroke at 1 year: 5.1% vs. 2.4% (p=0.07)
- Major vascular complications at 30 days: 11% vs. 3.2% (p<0.001)
Compared with TAVI patients, AVR patients had more episodes of major bleeding (9.3% vs. 19.5%, p<0.001) and new-onset AF (8.6% vs. 16%, p=0.006). At 30 days, more TAVI patients than AVR patients had an improvement in symptoms, but this difference was no longer significant at 1 year.
The authors concluded that TAVI “is an alternative to surgical replacement in a well-chosen, high-risk subgroup of patients with aortic stenosis. In the absence of long-term follow-up data, recommendations to individual patients must balance the appeal of avoiding the known risks of open-heart surgery against the less invasive transcatheter approach, which has different and less well understood risks, particularly with respect to stroke.”
In an accompanying editorial, Hartzell Schaff considers the implications of the increased risk of stroke observed in the trial. He writes that it is “not surprising that neurologic complications occur” with TAVI because “calcific atherosclerotic emboli are common during catheter and device manipulation of a stenotic aortic valve.” He says that “continued surveillance of patients… will be critically important to determine the durability of the transcatheter prosthesis and to assess the risk of late thromboembolic events.”
June 2nd, 2011
FDA: ARBs Don’t Increase Risk of Cancer
Larry Husten, PHD
Concluding a nearly one-year safety review, the FDA has announced that angiotensin receptor blockers (ARBs) do not increase the risk of cancer. The FDA initiated the review after a meta-analysis published in Lancet Oncology found a small but statistically significant increase in the risk of cancer among people taking ARBs. The FDA meta-analysis included 31 trials that randomized 156,000 patients.
“The FDA has completed its review of controlled trial data on more than 155,000 patients randomized to ARBs or other treatments — the largest evaluation of such data to date — and finds no evidence of an increased risk of cancer in patients who take an ARB,” said Mary Ross Southworth, Pharm. D., deputy director for safety in the Division of Cardiovascular and Renal Drugs in the FDA’s Center for Drug Evaluation and Research, in an FDA press release.
June 2nd, 2011
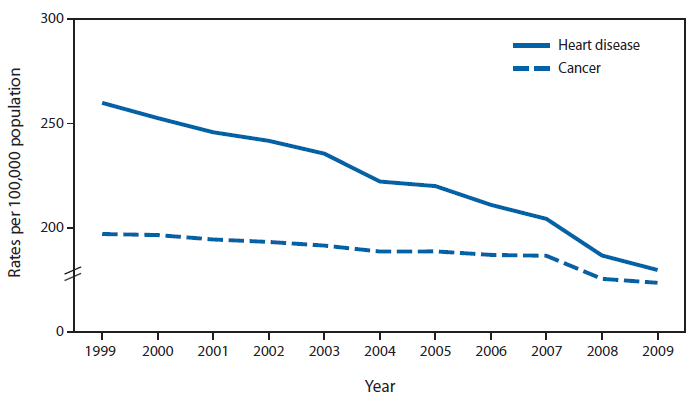
CDC: Death Rates for Heart Disease and Cancer Converge
Larry Husten, PHD
Preliminary data released by the CDC shows that the age-adjusted death rates for heart disease and cancer are converging dramatically. From 1999 through 2009, the death rates for heart disease and cancer declined by 30.8% and 11.9%, respectively. In 1999, the risk for death from heart disease was 31.9% higher than from cancer. By 2009, the difference had shrunk to only 3.6%. Here’s the CDC chart:
(Click to enlarge)
June 2nd, 2011
Small Study Suggests Possible Benefits of Fish-Oil Supplements for PCI Patients
Larry Husten, PHD
A small study from Poland raises the possibility that supplements containing omega-3 fatty acids may have beneficial effects when given to PCI patients already taking aspirin and clopidogrel. The investigator-initiated study randomized 54 PCI patients to either 1 g per day of n-3 PUFA daily or placebo for one month. The results have been published in Arteriosclerosis, Thrombosis, and Vascular Biology.
After one month, treatment with n-3-PUFA compared with placebo resulted in:
- significantly larger pores in the fibrin network, suggesting increased susceptibility to fibrinolysis,
- a reduction in thrombin generation, and
- a reduction in oxidative stress.
The supplements had no significant effect on fibrinogen or clotting-factor levels. A previous paper from the same study published last year in JACC reported that the fish-oil supplement helped augment the response of platelets to clopidogrel.
“There are no other studies on omega-3 effects in patients who were already being treated with optimal medical therapy after stent placement,” said the first author of the study, Grzegorz Gajos, in an AHA press release. “This was a proof of concept study. We were looking for any effect and what it might be.”
The authors concluded that their results indicate “novel antithrombotic effects produced by these agents. These effects may contribute to a decreased risk of thrombotic events after PCI following n-3 PUFA administration. A larger study is needed to assess clinical benefits potentially related to these novel antithrombotic effects of n-3 PUFA.”
June 1st, 2011
More Questions Raised About Biotronik’s Relationships with Cardiologists
Larry Husten, PHD
A story by Barry Meier in the New York Times provides new details about a widening investigation into the suspiciously close relationship of referring cardiologists and implanting electrophysiologists with the upstart heart device manufacturer Biotronik. The story cites examples of exchanges between Biotronik employees and their dealings with the physicians. According to Meier, the company is also the subject of a Justice Department investigation.
In one email, a Biotronik executive writes to his colleagues that in order to sell more pacemakers and defibrillators the company will need to fund unnecessary clinical trials to help physicians “generate income from research fees.” Referring cardiologists, who are called “feeders” in one of the documents, can receive as much as $4,800 for every patient they enroll in a company-financed study, Meier reports.
A lawyer for Biotronik told Meier that the company resisted pressure to fund “unscientific studies” and that all of the studies performed by the company were “scientifically sound.”
One former Biotronik employee provided the Times with numerous documents demonstrating how the company influenced physicians in Tucson, Arizona to dramatically increase their use of Biotronik products. One referring cardiologist told an electrophysiologist that he would not refer patients to him unless he started using Biotronik devices. Another electrophysiologist who accepted referrals from the cardiologist increased his usage of Biotronik products eightfold, reaching $1.1 million in 1 year.
A California electrophysiologist appeared caught between Biotronik and Medtronic. He told a Biotronik employee that his referring cardiologists insisted that he use Medtronic devices. The Biotronik official wrote his colleagues that this physician “candidly stated it is the referring community that must accept Biotronik in order for him to implant with us.”
In a previous article, the Times Barry Meier reported that several electrophysiologists in Las Vegas dramatically increased their usage of Biotronik products after becoming paid Biotronik consultants.
May 27th, 2011
AIM-HIGH Halted: A Death Knell for the HDL Hypothesis? Six Experts Weigh In
CardioExchange Editors, Staff
Earlier this week, the National Heart, Lung and Blood Institute stopped the randomized clinical trial known as AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health). It had been designed to test the addition of high-dose, extended-release niacin to statins in people who were at risk for cardiovascular events, had well-controlled LDL levels, but also had low HDL and elevated triglyceride levels (See CardioExchange’s news item describing details about the trial and the decision to halt it.)
We at CardioExchange put this question to one of the principal investigators of AIM-HIGH and to five other experts from different domains within the cardiovascular-medicine and scientific communities:
Is the halting of AIM-HIGH the death knell for the “HDL hypothesis”: the idea that clinical benefit can be achieved by raising HDL-cholesterol levels?
Here’s how our interviewees responded. We urge you, as members of the CardioExchange community, to engage them — and one another — in dialogue about this important research development.
No, emphatically, this trial does not sound the death knell for the HDL hypothesis. VA-HIT clearly validated that one can reduce death, MI, and stroke with gemfibrozil 12 to 13 years ago, absent any LDL change. But the baseline LDL in that earlier study was 111 mg/dL; in AIM-HIGH it was 71 mg/dL — a huge difference of 40 mg/dL. We must continue to pursue research in higher-risk ACS and acute-MI patients whose HDL levels are low — and perhaps include more de novo or statin-naive patients, as we often see in real-world practice. —William E. Boden, MD, co–principal investigator of the AIM-HIGH trial; Medical Director of Cardiovascular Services, Kaleida Health; Chief of Cardiology, Buffalo General Hospital and Millard Fillmore Gates Circle Hospital; Professor of Medicine and Preventive Medicine, University at Buffalo
Another one bites the dust! As I have maintained before, HDL is a very complex molecule that is involved in many metabolic pathways. Simply modifying the molecule qualitatively or quantitatively is not ipso facto going to translate into cardioprotective effects. It is hard to argue against a randomized, controlled outcomes-based trial as being an important tool to bust myths and entrenched beliefs! —Sanjay Kaul, MD, Director, Cardiology Fellowship Training Program, Cedars-Sinai Medical Center, Los Angeles; Professor of Medicine, UCLA School of Medicine
The discontinuation of this trial is disappointing. Of course, judging from a press release alone is extremely difficult for someone not associated with the study. Did the complexity of the relatively small study population of 1718 Niaspan-treated participants — heterogeneous risk factors (metabolic syndrome, high blood pressure, diabetes) and varying drug treatments (e.g., with and without ezetimibe) — influence the conclusion that niacin was not effective in preventing heart attacks and strokes? If so, how? (It’s somewhat surprising to see the ILLUMINATE trial mentioned in the NIH press release without noting that off-target effects are likely to have played a major role in that study’s results.) —Monty Krieger, PhD, Whitehead Professor of Molecular Genetics, Department of Biology, Massachusetts Institute of Technology (basic-science researcher who discovered the first HDL receptor)
The hypothesis is not dead, but it is certainly on life support. It is clear that HDL “raising” is too simplistic and that HDL manipulation is a more appropriate potential target. Nevertheless, the enticing combination of niacin added to statin therapy to improve cardiovascular risk has been dealt a serious blow. It can be resurrected only by an enticing subgroup analysis from AIM-HIGH or by a clear benefit demonstrated in HPS2-THRIVE. —Amit Khera, MD, MSc, Assistant Professor, UT Southwestern Medical Center
The HDL hypothesis may still be viable from the standpoint of future research, but this study is another disappointment to all of us who hope to further improve outcomes for patients at risk for cardiovascular disease. From the perspective of contemporary clinical care, we must step back and recognize that the evidence for lipid-modifying therapy, in terms of meaningful patient outcomes, supports only the use of statins, with little current role for agents that raise HDL. Other lipid-modifying therapies, including niacin (which failed in AIM-HIGH) and fibrates simply should not be used regularly, if at all. My perspective would change if a study or studies showed that lipid-modifying therapies that raise HDL have important clinical benefits beyond improving a patient’s lab-test result. —Frederick A. Masoudi, MD, MSPH, Associate Professor of Medicine, University of Colorado Denver; Denver Health Medical Center
This study does not overturn the strong evidence from animal models that some forms of HDL raising are likely beneficial. Nor does it prove that other approaches such as CETP inhibition, which involve both LDL lowering and HDL elevation, will fail. AIM-HIGH showed that the HDL-raising effect of extended-release niacin does not benefit patients whose LDL is tightly controlled at 40 to 80 mg/dL. This could indicate that the anti-atherogenic effects of HDL are not important in the absence of an ongoing atherogenic stimulus. Or it may imply that the particular mechanism of HDL elevation by niacin, which is unknown, is not beneficial. The data highlight, once again, the importance of testing specific hypotheses in carefully designed clinical trials. —Alan R. Tall, MD, Tilden Weger Bieler Professor of Medicine, Columbia University School of Medicine