An ongoing dialogue on HIV/AIDS, infectious diseases,
May 27th, 2015
START is STOPPED: Study Confirms HIV Treatment Is Beneficial for All, Even Those with High CD4 Cell Counts
The Strategic Timing of AntiRetroviral Treatment (START) study began in 2009, enrolling over 4000 asymptomatic people with HIV and CD4 cell counts > 500, and randomizing them to immediate ART or to wait until the count dropped to 350. Now, from the National Institute of Allergy and Infectious Diseases comes this important announcement:
Though the study was expected to conclude at the end of 2016, an interim review of the study data by an independent data and safety monitoring board (DSMB) recommended that results be released early… Based on data from March 2015, the DSMB found 41 instances of AIDS, serious non-AIDS events or death among those enrolled in the study’s early treatment group compared to 86 events in the deferred treatment group. The DSMB’s interim analysis found risk of developing serious illness or death was reduced by 53 percent among those in the early treatment group, compared to those in the deferred group.
So now we have it — definitive evidence that it’s better to be on HIV treatment than to wait, even for those with normal CD4 cell counts and no symptoms.
It’s worth revisiting, just for history’s sake, why the START study (which opened in 2009) was even done. Remember that once upon a time — OK, a bit more than a decade ago — we tried to wait long as possible before starting someone with HIV who was asymptomatic on antiretroviral therapy. Wait until CD4 = 350? Or even 200? No big deal, provided they had no HIV-related symptoms and were closely monitored.
Seems impossible now — how could we have done such a thing? A bunch of reasons, most (all) of them irrelevant or disproven over time:
- HIV treatment had short and long-term side effects, some of them potentially severe. This was the primary motivation to wait, and actually quite true in the AZT, d4T, ddI, indinavir, etc., era. Of course much less so now. But in the era of lipoatrophy, lactic acidosis, lipoatrophy, high pill burdens, and GI side effects, it made sense to wait if possible.
- We thought there was no downside to waiting, since the immune function (as measured by CD4 cell counts) would return to safe levels after starting ART. Unfortunately this recovery doesn’t always occur, plus we now know there was irreversible loss of immune function based on CD4 nadir.
- Viral replication without loss of CD4 cells was viewed as benign. The potentially deleterious effects of immune activation and inflammation were barely considered, especially before we know the results of the (similarly named) SMART study of intermittent therapy.
- If we treated “early,” then options for therapy would become limited due to resistance — including when patients really need ART (i.e., have low CD4 cell counts). This view was based on the assumption that treatment failure with resistance was inevitable — turns out it’s not. We furthermore didn’t know that the late 2000s would bring a spectacular flurry of drug development for patients with resistant virus. Finally, it ignored the fact that the best way to avoid having a low CD4 was to not let it drop in the first place!
- No clinical trial proved that waiting was harmful for patients with high CD4 cell counts. Over time, there were three randomized studies supporting earlier therapy (one done in Haiti, the clinical outcomes analysis of HPTN 052, and more recently TEMPRANO) — and a fourth if you count the SMART “naive” analysis. However, doubters maintained that most of these data (SMART excluded) were collected in resource-limited settings, and/or used CD4 thresholds that were too low.
- The full benefit of HIV treatment as prevention was not fully appreciated until HPTN 052. Even though indirect data strongly suggested that HIV treatment would reduce viral transmission, it wasn’t until the results of HPTN 052 became available in 2011 that this extraordinary advantage of being on suppressive therapy really hit home, both for providers and patients. This completely changed the dialogue in the clinic — now asymptomatic people with HIV want to be on therapy, for obvious reasons.
The above advances in knowledge have meant that in the United States, HIV treatment guidelines have recommended that all patients with HIV be treated for several years — specifically:
Antiretroviral therapy (ART) is recommended for all HIV-infected individuals to reduce the risk of disease progression … ART also is recommended for HIV-infected individuals for the prevention of transmission of HIV.
It couldn’t be clearer, but to put it in the way familiar to anyone who’s taught from any “HIV 101” slide set, here you go:
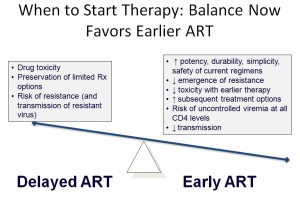
So while the results of the START study are important — and when the full analysis is released, will be fascinating — the study results will not have much of an impact here. Pretty much everyone in care is on therapy already.
The study will, however, have substantial implications globally, where deferring therapy remains a common strategy for patients with high CD4 cell counts.
And score one for Alice Pau and the prescient DHHS Guidelines — they first made this call in 2012. She won’t say, “I told you so,” so I’ll say it for her!


Nice summary, thank you Paul. As you suggest, based on other recent data I’m not surprised that waiting for the CD4 to drop to 350 engendered poorer outcomes. However, I’d like to see a subgroup analysis of patients not on ART whose CD4s remained >500 compared to matched controls who took ART, and whether or not that will strengthen the current BIII recommendation for this group when the guidelines are updated. It would be nice to take some of the nuance out of this distinction and be able to say that ART is an A1 recommendation for all.
Dear Paul; Thanks for this post.
It seems to be a wise step forward and is following the same trend that HCV treatment guidelines have and even with the same less or more reasons: ARVs introduction to the market in HIV history has moved from high toxicity & low potency ARVs to high toxicity & high potency stage and finally low toxicity & high potency period. Specially with the introduction of entry inhibitors and integrase inhibitors which has very low side effects, the fear from side effects are no more a big concern (UNAIDS Gap Report 2015). This is again specially wise, as entry inhibitors are more effective on R5 HIV viruses which are found more in the naive, not- ARV exposed patients.
The same reasons made a big revolution in HCV treatment guidelines in recent years too.
Best,
Hossain
This is some good news. I hope governments world over will prioritize voluntary testing so one can be on medication early enough. Thank you for the information.
Nice, concise summary. Well–evidence-based medicine takes some time. Let’s not be quite so self-congratulatory though. Those of us of a certain age remember the ravages of early ART and the price of resistance.
Cheers.
Thanks for the summary of these important results. Lets hope it doesn’t take so long to shift the tide towards treatment for all for children!
This is some good news. I hope governments world over will prioritize voluntary testing so one can be on medication early enough. Thank you for the information.