An ongoing dialogue on HIV/AIDS, infectious diseases,
January 29th, 2016
Elbasvir/Grazoprevir Combination Pill for HCV a Welcome New Option — With a Few Buts
 As expected, there’s a new option for HCV therapy, the combination pill elbasvir/grazoprevir (EBR/GZR, brand name Zepatier, more on this below), and it’s indicated for genotypes 1 and 4. For those mechanistically inclined, elbasvir is an NS5A inhibitor (like ledipasvir), and grazoprevir is a protease inhibitor (like simeprevir).
As expected, there’s a new option for HCV therapy, the combination pill elbasvir/grazoprevir (EBR/GZR, brand name Zepatier, more on this below), and it’s indicated for genotypes 1 and 4. For those mechanistically inclined, elbasvir is an NS5A inhibitor (like ledipasvir), and grazoprevir is a protease inhibitor (like simeprevir).
This is the second one-pill, once a day option for HCV, and unlike the first (ledipasvir/sofosbuvir, or LDV/SOF), it can be used in patients with end-stage renal disease on hemodialysis — an advantage that granted the regimen a “breakthrough therapy” designation, speeding FDA approval.
In addition, the list price is substantially lower — $54,000 for 12 weeks of EBR/GZR, vs $94,000 for LDV/SOF. Of course the actual price paid by government and private payers is heavily negotiated, so how the two will compare remains to be seen. And some providers are treating their low viral load genotype 1 patients with just 8 weeks of LDV/SOF therapy, bringing the price much closer to $54,000 (at least in those cases).
But why the lower price? After all, cure rates are outstanding (94-97% for genotype 1), side effect rates low, and you can’t get simpler than one pill a day.
Seems to me there are two major reasons:
- They’re not the first. LDV/SOF has been available for over a year, and providers are incredibly comfortable with it. Plus, you can barely take a walk down the street, turn on the radio in your car, or flip on the TV without encountering a direct-to-patient advertisement for LDV/SOF. This lower price will be an important strategy for convincing providers, patients, and payers that EBR/GZP is an option.
- It’s not quite as convenient as LDV/SOF. A patient starting HCV with EBR/GZR is somewhat less likely to be taking just one pill a day for 12 weeks than if treated with LDV/SOF.
The reason for this slightly greater complexity is that one of the predictors of treatment failure in studies of EBR/GZR was the pre-treatment presence of certain NS5A polymorphisms — which are similar to resistance mutations:
Providers will need to do a pre-treatment test for these polymorphisms. Based on these results and the treatment history, here are the treatment recommendations for the EBR/GZR regimen:
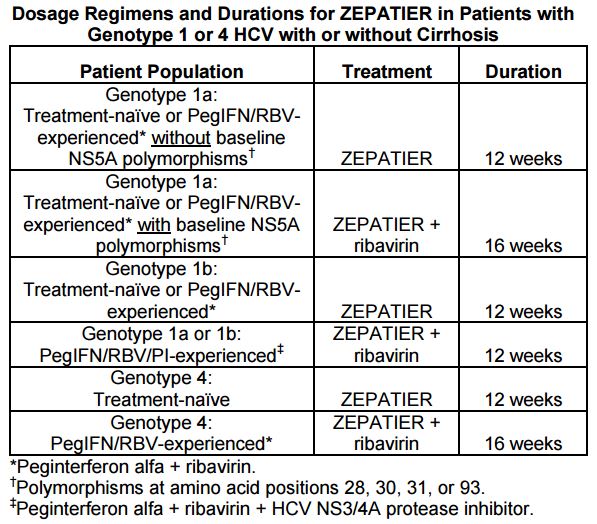
And how common are the polymorphisms? Per the package insert, “The prevalence of polymorphisms at any of these positions in genotype 1a-infected subjects was 11% (62/561) overall, and 12% (37/309) specifically for subjects in the U.S. across Phase 2 and Phase 3 clinical trials …”
Furthermore, as noted above, some of the regimens will also need to be extended to 16 weeks, and the prescribing information recommends LFT monitoring during treatment for all patients. Drug-drug interactions are also more common than with LDV/SOF.
These issues notwithstanding, EBR/GZR is a simpler treatment than the “PrOD” regimen (paritaprevir, ritonavir, ombitasvir, and dasabuvir) — and for the record less expensive than that one, too.
Not only that, the brand name (Zepatier!) offers a nice French souffle to our existing Italian baked pasta with cheese (Harvoni!).

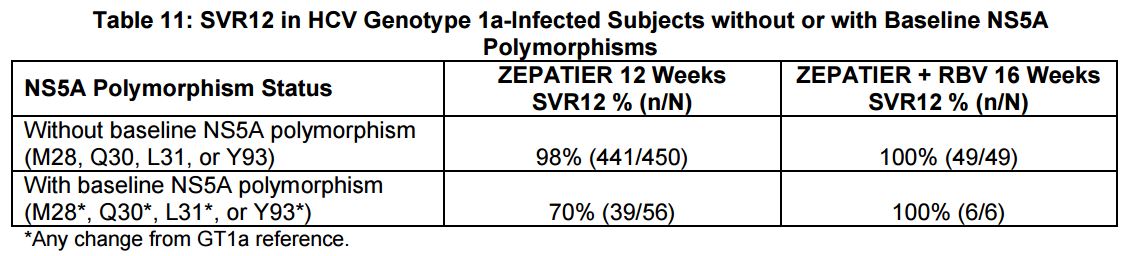

Paul, reviewing the package insert summary today, revealed the fact that the FDA did not include all pertinent info into the first summary page (pt population-duration chart) in terms of how long the mnf recommends treating G1a, experienced, had been on PEG/RBN/ AND a 1st gen PI. They do mention this in the BODY of the package insert under dosage recommendations though, but not in the first summary where most providers probably look…and this happens a lot i.e. they at times leave info out of the 1st summary, below is in the body of the PI.
Genotype 1a§ or 1b: PegIFN/RBV/PI-experienced¶ ZEPATIER + RBV‡ 12 weeks
§The optimal ZEPATIER-based treatment regimen and duration of therapy for PegIFN/RBV/PI-experienced genotype 1a-infected patients with one or more baseline NS5A resistance-associated polymorphisms at positions 28, 30, 31,
and 93 has not been established.
This is the difference of another 4 weeks of cost/side effects. Is this because there is simply no data?